Meningitis is a clinical syndrome characterized by inflammation of the meninges. The image below depicts acute bacterial meningitis.
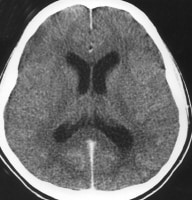 Acute bacterial meningitis. This axial nonenhanced computed tomography scan shows mild ventriculomegaly and sulcal effacement.
Acute bacterial meningitis. This axial nonenhanced computed tomography scan shows mild ventriculomegaly and sulcal effacement.Signs and symptoms
The classic triad of bacterial meningitis consists of the following:
- Fever
- Headache
- Neck stiffness
Other symptoms can include nausea, vomiting, photalgia (photophobia), sleepiness, confusion, irritability, delirium, and coma. Patients with viral meningitis may have a history of preceding systemic symptoms (eg, myalgias, fatigue, or anorexia).
The history should also address the following:
- Epidemiologic factors and predisposing risks
- Exposure to a patients or animals with a similar illness
- Previous medical treatment and existing conditions
- Geographic location and travel history
- Season and temperature
Acute bacterial meningitis in otherwise healthy patients who are not at the extremes of age presents in a clinically obvious fashion; however, subacute bacterial meningitis often poses a diagnostic challenge.
General physical findings in viral meningitis are common to all causative agents. Enteroviral infection is suggested by the following:
- Exanthemas
- Symptoms of pericarditis, myocarditis, or conjunctivitis
- Syndromes of pleurodynia, herpangina, and hand-foot-and-mouth disease
Infants may have the following:
- Bulging fontanelle (if euvolemic)
- Paradoxic irritability (ie, remaining quiet when stationary and crying when held)
- High-pitched cry
- Hypotonia
The examination should evaluate the following:
- Focal neurologic signs
- Signs of meningeal irritation
- Systemic and extracranial findings
- Chronic meningitis
In chronic meningitis, it is essential to perform careful general, systemic, and neurologic examinations, looking especially for the following:
- Lymphadenopathy
- Papilledema and tuberculomas during funduscopy
- Meningismus
- Cranial nerve palsies
Patients with aseptic meningitis syndrome usually appear clinically nontoxic, with no vascular instability. They characteristically have an acute onset of meningeal symptoms, fever, and CSF pleocytosis that is usually prominently lymphocytic.
See Clinical Presentation for more detail.
Diagnosis
The diagnostic challenges in patients with clinical findings of meningitis are as follows:
- Early identification and treatment of patients with acute bacterial meningitis
- Assessing whether a treatable CNS infection is present in those with suspected subacute or chronic meningitis
- Identifying the causative organism
Blood studies that may be useful include the following:
- Complete blood count (CBC) with differential
- Serum electrolytes
- Serum glucose (which is compared with the CSF glucose)
- Blood urea nitrogen (BUN) or creatinine and liver profile
In addition, the following tests may be ordered:
- Blood, nasopharynx, respiratory secretion, urine or skin lesion cultures
- Syphilis testing
- Serum procalcitonin testing
- Lumbar puncture and CSF analysis
- Neuroimaging (CT of the head and MRI of the brain)
See Workup for more detail.
Management
Initial measures include the following:
- Shock or hypotension – Crystalloids
- Altered mental status – Seizure precautions and treatment (if necessary), along with airway protection (if warranted)
- Stable with normal vital signs – Oxygen, IV access, and rapid transport to the emergency department (ED)
Treatment of bacterial meningitis includes the following:
- Prompt initiation of empiric antibacterial therapy as appropriate for patient age and condition
- After identification of the pathogen and determination of susceptibilities, targeted antibiotic therapy as appropriate for patient age and condition
- Steroid (typically, dexamethasone) therapy
- In patients with nosocomial meningitis, intrathecal antibiotics
The following systemic complications of acute bacterial meningitis must be treated:
- Hypotension or shock
- Hypoxemia
- Hyponatremia
- Cardiac arrhythmias and ischemia
- Stroke
- Exacerbation of chronic diseases
Most cases of viral meningitis are benign and self-limited, but in certain instances, specific antiviral therapy may be indicated, if available.
Other types of meningitis are treated with specific therapy as appropriate for the causative pathogen, as follows:
- Fungal meningitis - Cryptococcal (amphotericin B, flucytosine, fluconazole, itraconazole), Coccidioides immitis (fluconazole, intrathecal amphoytericin B, itraconazole), Histoplasma capsulatum (liposomal amphotericin B, itraconazole), or Candida (IM or aqueous penicillin G, probenecid)
- Tuberculous meningitis (isoniazid, rifampin, pyrazinamide, ethambutol, streptomycin)
- Parasitic meningitis – Amebic (amphotericin B, miconazole, rifampin) or helminthic (largely supportive)
- Lyme meningitis (ceftriaxone; alternatively, penicillin G, doxycycline, chloramphenicol)
See Treatment and Medication for more detail.
Background
Infections of the central nervous system (CNS) can be divided into 2 broad categories: those primarily involving the meninges (meningitis; see the image below) and those primarily confined to the parenchyma (encephalitis).
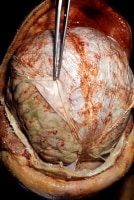 Pneumococcal meningitis in a patient with alcoholism. Courtesy of the CDC/Dr. Edwin P. Ewing, Jr.
Pneumococcal meningitis in a patient with alcoholism. Courtesy of the CDC/Dr. Edwin P. Ewing, Jr.
Meningitis is a clinical syndrome characterized by inflammation of the meninges, the 3 layers of membranes that enclose the brain and spinal cord. These layers consist of the following:
- Dura - A tough outer membrane
- Arachnoid - A lacy, weblike middle membrane
- Subarachnoid space - A delicate, fibrous inner layer that contains many of the blood vessels that feed the brain and spinal cord
Risk factors for meningitis include the following:
- Extremes of age (< 5 or >60 years)
- Diabetes mellitus, renal or adrenal insufficiency, hypoparathyroidism, or cystic fibrosis
- Immunosuppression, which increases the risk of opportunistic infections and acute bacterial meningitis
- HIV infection, which predisposes to bacterial meningitis caused by encapsulated organisms, primarily Streptococcus pneumoniae, and opportunistic pathogens
- Crowding (such as that experienced by military recruits and college dorm residents), which increases the risk of outbreaks of meningococcal meningitis
- Splenectomy and sickle cell disease, which increase the risk of meningitis secondary to encapsulated organisms
- Alcoholism and cirrhosis
- Recent exposure to others with meningitis, with or without prophylaxis
- Contiguous infection (eg, sinusitis)
- Dural defect (eg, traumatic, surgical, or congenital)
- Thalassemia major
- Intravenous (IV) drug abuse
- Bacterial endocarditis
- Ventriculoperitoneal shunt
- Malignancy (increased risk of Listeria infection)
- Some cranial congenital deformities
Clinically, meningitis manifests with meningeal symptoms (eg, headache, nuchal rigidity, or photophobia), as well as pleocytosis (an increased number of white blood cells [WBCs]) in the cerebrospinal fluid (CSF). Depending on the duration of symptoms, meningitis may be classified as acute or chronic. (See Etiology and Presentation.)
Anatomically, meningitis can be divided into inflammation of the dura (sometimes referred to as pachymeningitis), which is less common, and leptomeningitis, which is more common and is defined as inflammation of the arachnoid tissue and subarachnoid space. (See Anatomy.)
Meningitis can also be divided into the following 3 general categories:
- Bacterial (pyogenic)
- Granulomatous
- Aseptic
The most common cause of meningeal inflammation is irritation caused by bacterial or viral infections. The organisms usually enter the meninges through the bloodstream from other parts of the body. Most cases of bacterial meningitis are localized over the dorsum of the brain; however, under certain conditions, meningitis may be concentrated at the base of the brain, as with fungal diseases and tuberculosis. (See Etiology.)
Bacterial meningitis consists of pyogenic inflammation of the meninges and the underlying subarachnoid CSF. If not treated, it may lead to lifelong disability or death. Before the antimicrobial era, bacterial meningitis was uniformly fatal, but with the advent of antimicrobial therapy, the overall mortality from this disease has decreased. Nonetheless, it remains alarmingly high: approximately 25%. (See Epidemiology.)
The emergence of resistant bacterial strains has prompted changes in antibiotic protocols in some countries, including the United States. Apart from dexamethasone, neuronal cell protectants still hold only future promise as adjunctive therapy. (See Treatment and Medication.)
The specific infectious agents that are involved in bacterial meningitis vary among different patient age groups, and the inflammation may evolve into the following conditions:
- Ventriculitis
- Empyema
- Cerebritis
- Abscess formation
Meningitis can also be also classified more specifically according to its etiology. Numerous infectious and noninfectious causes of meningitis have been identified. Examples of common noninfectious causes include medications (eg, nonsteroidal anti-inflammatory drugs [NSAIDs] and antibiotics) and carcinomatosis. (See Etiology.)
Bacterial meningitis
Acute bacterial meningitis denotes a bacterial cause of this syndrome. This is usually characterized by an acute onset of meningeal symptoms and neutrophilic pleocytosis. Depending on the specific bacterial cause, the syndrome may be called, for example, any of the following:
- Pneumococcal meningitis
- Haemophilus influenzae meningitis
- Staphylococcal meningitis
- Meningococcal meningitis
- Tuberculous meningitis
- Pediatric bacterial meningitis
Chronic meningitis is a constellation of signs and symptoms of meningeal irritation associated with CSF pleocytosis that persists for longer than 4 weeks.
Unlike subacute (developing over 1-7 days) or chronic (>7 days) meningitis, which have myriad infectious and noninfectious etiologies, acute meningitis (< 1 day) is almost always a bacterial infection caused by 1 of several organisms. Depending on age and general condition, these gravely ill patients present acutely with signs and symptoms of meningeal inflammation and systemic infection of less than 24 hours’ (and usually >12 hours’) duration.
Patients with acute bacterial meningitis may decompensate very quickly. Consequently, they require emergency care, including the administration of appropriate antimicrobial therapy as soon as possible once bacterial meningitis is suspected or proven.
Nonbacterial meningitis
Fungal and parasitic forms of meningitis are also named according to their specific etiologic agent (eg, cryptococcal meningitis, Histoplasma meningitis, and amebic meningoencephalitis).
In many cases, a cause of meningitis is not apparent after initial evaluation, and the disease is therefore classified as aseptic meningitis. These patients characteristically have an acute onset of meningeal symptoms, fever, and CSF pleocytosis that is usually prominently lymphocytic.
When the cause of aseptic meningitis is discovered, the disease can be reclassified according to its etiology. If appropriate diagnostic methods are performed, a specific viral etiology is identified in 55-70% of cases of aseptic meningitis. However, the condition can also be caused by bacterial, fungal, mycobacterial, and parasitic agents.
If, after an extensive workup, aseptic meningitis is found to have a viral etiology, it can be reclassified as a form of acute viral meningitis (eg, enteroviral meningitis or herpes simplex virus [HSV] meningitis).
Pathophysiology
Most cases of meningitis are caused by an infectious agent that has colonized or established a localized infection elsewhere in the host. Potential sites of colonization or infection include the skin, the nasopharynx, the respiratory tract, the gastrointestinal (GI) tract, and the genitourinary tract. The organism invades the submucosa at these sites by circumventing host defenses (eg, physical barriers, local immunity, and phagocytes or macrophages).
An infectious agent (ie, a bacterium, virus, fungus, or parasite) can gain access to the CNS and cause meningeal disease via any of the 3 following major pathways:
- Invasion of the bloodstream (ie, bacteremia, viremia, fungemia, or parasitemia) and subsequent hematogenous seeding of the CNS
- A retrograde neuronal (eg, olfactory and peripheral nerves) pathway (eg,Naegleria fowleri or Gnathostoma spinigerum)
- Direct contiguous spread (eg, sinusitis, otitis media, congenital malformations, trauma, or direct inoculation during intracranial manipulation)
Invasion of the bloodstream and subsequent seeding is the most common mode of spread for most agents. This pathway is characteristic of meningococcal, cryptococcal, syphilitic, and pneumococcal meningitis.
Rarely, meningitis arises from invasion via septic thrombi or osteomyelitic erosion from infected contiguous structures. Meningeal seeding may also occur with a direct bacterial inoculate during trauma, neurosurgery, or instrumentation. Meningitis in the newborn may be transmitted vertically, involving pathogens that have colonized the maternal intestinal or genital tract, or horizontally, from nursery personnel or caregivers at home.
Local extension from contiguous extracerebral infection (eg, otitis media, mastoiditis, or sinusitis) is a common cause. Possible pathways for the migration of pathogens from the middle ear to the meninges include the following:
- The bloodstream
- Preformed tissue planes (eg, posterior fossa)
- Temporal bone fractures
- The oval or round window membranes of the labyrinths
The brain is naturally protected from the body’s immune system by the barrier that the meninges create between the bloodstream and the brain. Normally, this protection is an advantage because the barrier prevents the immune system from attacking the brain. However, in meningitis, the blood-brain barrier can become disrupted; once bacteria or other organisms have found their way to the brain, they are somewhat isolated from the immune system and can spread.
When the body tries to fight the infection, the problem can worsen; blood vessels become leaky and allow fluid, WBCs, and other infection-fighting particles to enter the meninges and brain. This process, in turn, causes brain swelling and can eventually result in decreasing blood flow to parts of the brain, worsening the symptoms of infection.[3]
Depending on the severity of bacterial meningitis, the inflammatory process may remain confined to the subarachnoid space. In less severe forms, the pial barrier is not penetrated, and the underlying parenchyma remains intact. However, in more severe forms of bacterial meningitis, the pial barrier is breached, and the underlying parenchyma is invaded by the inflammatory process. Thus, bacterial meningitis may lead to widespread cortical destruction, particularly when left untreated.
Replicating bacteria, increasing numbers of inflammatory cells, cytokine-induced disruptions in membrane transport, and increased vascular and membrane permeability perpetuate the infectious process in bacterial meningitis. These processes account for the characteristic changes in CSF cell count, pH, lactate, protein, and glucose in patients with this disease.
Exudates extend throughout the CSF, particularly to the basal cisterns, resulting in the following:
- Damage to cranial nerves (eg, cranial nerve VIII, with resultant hearing loss)
- Obliteration of CSF pathways (causing obstructive hydrocephalus)
- Induction of vasculitis and thrombophlebitis (causing local brain ischemia)
Intracranial pressure and cerebral fluid
One complication of meningitis is the development of increased intracranial pressure (ICP). The pathophysiology of this complication is complex and may involve many proinflammatory molecules as well as mechanical elements. Interstitial edema (secondary to obstruction of CSF flow, as in hydrocephalus), cytotoxic edema (swelling of cellular elements of the brain through the release of toxic factors from the bacteria and neutrophils), and vasogenic edema (increased blood brain barrier permeability) are all thought to play a role.
Without medical intervention, the cycle of decreasing CSF, worsening cerebral edema, and increasing ICP proceeds unchecked. Ongoing endothelial injury may result in vasospasm and thrombosis, further compromising CSF, and may lead to stenosis of large and small vessels. Systemic hypotension (septic shock) also may impair CSF, and the patient soon dies as a consequence of systemic complications or diffuse CNS ischemic injury.
Cerebral edema
The increased CSF viscosity resulting from the influx of plasma components into the subarachnoid space and diminished venous outflow lead to interstitial edema. The accumulation of the products of bacterial degradation, neutrophils, and other cellular activation leads to cytotoxic edema.
The ensuing cerebral edema (ie, vasogenic, cytotoxic, and interstitial) significantly contributes to intracranial hypertension and a consequent decrease in cerebral blood flow. Anaerobic metabolism ensues, which contributes to increased lactate concentration and hypoglycorrhachia. In addition, hypoglycorrhachia results from decreased glucose transport into the spinal fluid compartment. Eventually, if this uncontrolled process is not modulated by effective treatment, transient neuronal dysfunction or permanent neuronal injury results.
Cytokines and secondary mediators in bacterial meningitis
Key advances in understanding the pathophysiology of meningitis include insight into the pivotal roles of cytokines (eg, tumor necrosis factor alpha [TNF-α] and interleukin [IL]-1), chemokines (IL-8), and other proinflammatory molecules in the pathogenesis of pleocytosis and neuronal damage during occurrences of bacterial meningitis.
Increased CSF concentrations of TNF-α, IL-1, IL-6, and IL-8 are characteristic findings in patients with bacterial meningitis. Cytokine levels, including those of IL-6, TNF-α, and interferon gamma, have been found to be elevated in patients with aseptic meningitis.
The proposed events involving these inflammation mediators in bacterial meningitis begin with the exposure of cells (eg, endothelial cells, leukocytes, microglia, astrocytes, and meningeal macrophages) to bacterial products released during replication and death; this exposure incites the synthesis of cytokines and proinflammatory mediators. This process is likely initiated by the ligation of the bacterial components (eg, peptidoglycan and lipopolysaccharide) to pattern-recognition receptors, such as the Toll-like receptors (TLRs).
TNF-α and IL-1 are most prominent among the cytokines that mediate this inflammatory cascade. TNF-α is a glycoprotein derived from activated monocyte-macrophages, lymphocytes, astrocytes, and microglial cells.
IL-1, previously known as endogenous pyrogen, is also produced primarily by activated mononuclear phagocytes and is responsible for the induction of fever during bacterial infections. Both IL-1 and TNF-α have been detected in the CSF of individuals with bacterial meningitis. In experimental models of meningitis, they appear early during the course of disease and have been detected within 30-45 minutes of intracisternal endotoxin inoculation.
Many secondary mediators, such as IL-6, IL-8, nitric oxide, prostaglandins (eg, prostaglandin E2 [PGE2]), and platelet activation factor (PAF), are presumed to amplify this inflammatory event, either synergistically or independently. IL-6 induces acute-phase reactants in response to bacterial infection. The chemokine IL-8 mediates neutrophil chemoattractant responses induced by TNF-α and IL-1.
Nitric oxide is a free radical molecule that can induce cytotoxicity when produced in high amounts. PGE2, a product of cyclooxygenase (COX), appears to participate in the induction of increased blood-brain barrier permeability. PAF, with its myriad biologic activities, is believed to mediate the formation of thrombi and the activation of clotting factors within the vasculature. However, the precise roles of all these secondary mediators in meningeal inflammation remain unclear.
The net result of the above processes is vascular endothelial injury and increased blood-brain barrier permeability, leading to the entry of many blood components into the subarachnoid space. In many cases, this contributes to vasogenic edema and elevated CSF protein levels. In response to the cytokines and chemotactic molecules, neutrophils migrate from the bloodstream and penetrate the damaged blood-brain barrier, producing the profound neutrophilic pleocytosis characteristic of bacterial meningitis.
Genetic predisposition to inflammatory response
The inflammatory response and the release of proinflammatory mediators are critical to the recruitment of excess neutrophils to the subarachnoid space. These activated neutrophils release cytotoxic agents, including oxidants and metalloproteins that cause collateral damage to brain tissue.
Pattern recognition receptors, of which TLR A4 (TLRA4) is the best studied, lead to increase in the myeloid differentiation 88 (MyD88)-dependent pathway and excess production of proinflammatory mediators. At present, dexamethasone is used to decrease the effects of cellular toxicity by neutrophils after they are present. Researchers are actively seeking ways of inhibiting TLRA4 and other proinflammatory recognition receptors through genetically engineered suppressors.
Bacterial seeding
Bacterial seeding of the meninges usually occurs through hematogenous spread. In patients without an identifiable source of infection, local tissue and bloodstream invasion by bacteria that have colonized the nasopharynx may be a common source. Many meningitis-causing bacteria are carried in the nose and throat, often asymptomatically. Most meningeal pathogens are transmitted through the respiratory route, including Neisseria meningitidis (meningococcus) and S pneumoniae (pneumococcus).
Certain respiratory viruses are thought to enhance the entry of bacterial agents into the intravascular compartment, presumably by damaging mucosal defenses. Once in the bloodstream, the infectious agent must escape immune surveillance (eg, antibodies, complement-mediated bacterial killing, and neutrophil phagocytosis).
Subsequently, hematogenous seeding into distant sites, including the CNS, occurs. The specific pathophysiologic mechanisms by which the infectious agents gain access to the subarachnoid space remain unclear. Once inside the CNS, the infectious agents likely survive because host defenses (eg, immunoglobulins, neutrophils, and complement components) appear to be limited in this body compartment. The presence and replication of infectious agents remain uncontrolled and incite the cascade of meningeal inflammation described above.
Etiology
Causes of meningitis include bacteria, viruses, fungi, parasites, and drugs (eg, NSAIDs, metronidazole, and IV immunoglobulin [IVIg]). Certain risk factors are associated with particular pathogens.
HIV infection increases susceptibility to meningitis from a variety of pathogens, including cryptococci, Mycobacterium tuberculosis, syphilis, and Listeria species. In addition, HIV itself may cause aseptic meningitis (see Meningitis in HIV).
Other viral causes of meningitis include the following:
- Enteroviruses
- West Nile virus
- Human herpesvirus (HHV)-2
- Lymphocytic choriomeningitis virus (LCM)
In patients who have had trauma or neurosurgery, the most common microorganisms are S pneumoniae (if CSF leak is present), Staphylococcus aureus,enterobacteria, and Pseudomonas aeruginosa. In patients with an infected ventriculoperitoneal (atrial) shunt, the most common microorganisms areStaphylococcus epidermidis, S aureus, enterobacteria, Propionibacterium acnes,and diphtheroids (rare). Consultation with a neurosurgeon is indicated; early shunt removal is usually necessary for cure.
Pachymeningitis
As indicated by the presence of abundant pus, pachymeningitis most often results from a bacterial infection (usually staphylococcal or streptococcal) that is localized to the dura. The organisms most often gain access to the meninges via a skull defect (eg, a skull fracture) or spread from an infection of the paranasal sinuses or cranial osteomyelitis.
Haemophilus influenzae meningitis
H influenzae is a small, pleomorphic, gram-negative coccobacillus that is frequently found as part of the normal flora in the upper respiratory tract. The organism can spread from one individual to another in airborne droplets or by direct contact with secretions. Meningitis is the most serious acute manifestation of systemic infection with H influenzae. (See Haemophilus Meningitis.)
In the past, H influenzae was a major cause of meningitis, and the encapsulated type b strain of the organism (Hib) accounted for the majority of cases. Since the introduction of Hib vaccine in the United States in 1990, the overall incidence of H influenzae meningitis has decreased by 35%, with Hib accounting for fewer than 9.4% of H influenzae cases.
The isolation of H influenzae in adults suggests the presence of an underlying medical disorder, such as the following:
- Paranasal sinusitis
- Otitis media
- Alcoholism
- CSF leak after head trauma
- Functional or anatomic asplenia
- Hypogammaglobulinemia
Pneumococcal meningitis
S pneumoniae, a gram-positive coccus, is the most common bacterial cause of meningitis. In addition, it is the most common bacterial agent in meningitis associated with basilar skull fracture and CSF leak. It may be associated with other focal infections, such as pneumonia, sinusitis, or endocarditis (as, for example, in Austrian syndrome, which is the triad of pneumococcal meningitis, endocarditis, and pneumonia).
S pneumoniae is a common colonizer of the human nasopharynx; it is present in 5-10% of healthy adults and 20-40% of healthy children. It causes meningitis by escaping local host defenses and phagocytic mechanisms, either through choroid plexus seeding from bacteremia or through direct extension from sinusitis or otitis media.
Patients with the following conditions are at increased risk for S pneumoniaemeningitis:
- Hyposplenism
- Hypogammaglobulinemia
- Multiple myeloma
- Glucocorticoid treatment
- Defective complement (C1-C4)
- Diabetes mellitus
- Renal insufficiency
- Alcoholism
- Malnutrition
- Chronic liver disease
Streptococcus agalactiae meningitis
Streptococcus agalactiae (group B streptococcus [GBS]) is a gram-positive coccus that inhabits the lower GI tract. It also colonizes the female genital tract at a rate of 5-40%, which explains why it is the most common agent of neonatal meningitis (associated with 70% of cases).
Predisposing risks in adults include the following:
- Diabetes mellitus
- Pregnancy
- Alcoholism
- Hepatic failure
- Renal failure
- Corticosteroid treatment
In 43% of adult cases, however, no underlying disease is present.
Meningococcal meningitis
N meningitidis is a gram-negative diplococcus that is carried in the nasopharynx of otherwise healthy individuals. It initiates invasion by penetrating the airway epithelial surface. The precise mechanism by which this occurs is unclear, but recent viral or mycoplasmal infection has been reported to disrupt the epithelial surface and facilitate invasion by meningococcus.
Most sporadic cases of meningococcal meningitis (95-97%) are caused by serogroups B, C, and Y, whereas the A and C strains are observed in epidemics (< 3% of cases). Currently, N meningitidis is the leading cause of bacterial meningitis in children and young adults, accounting for 59% of cases.
Risk factors for meningococcal meningitis include the following:
- Deficiencies in terminal complement components (eg, membrane attack complex, C5-C9), which increases attack rates but is associated with surprisingly lower mortality rates
- Properdin defects that increase the risk of invasive disease
- Antecedent viral infection, chronic medical illness, corticosteroid use, and active or passive smoking
- Crowded living conditions, as is observed in college dormitories (college freshmen living in dormitories are at highest risk) and military facilities, which has been reported in clustering of cases
Listeria monocytogenes meningitis
Listeria monocytogenes is a small gram-positive bacillus that causes 3% of bacterial meningitis cases and is associated with one of the highest mortalities (20%).[5] The organism is widespread in nature and has been isolated in the stool of 5% of healthy adults. Most human cases appear to be food-borne.
L monocytogenes is a common food contaminant, with a recovery rate of up to 70% from raw meat, vegetables, and meats. Outbreaks have been associated with consumption of contaminated coleslaw, milk, cheese, and alfalfa tablets.
Groups at risk include the following:
- Pregnant women
- Infants and children
- Elderly individuals (>60 years)
- Patients with alcoholism
- Adults who are immunosuppressed (eg, steroid users, transplant recipients, or persons with AIDS)
- Individuals with chronic liver and renal disease
- Individuals with diabetes
- Persons with iron-overload conditions (eg, hemochromatosis or transfusion-induced iron overload)
Meningitis caused by gram-negative bacilli
Aerobic gram-negative bacilli include the following:
- Escherichia coli
- Klebsiella pneumoniae
- Serratia marcescens
- P aeruginosa
- Salmonella species
Gram-negative bacilli can cause meningitis in certain groups of patients. E coli is a common agent of meningitis among neonates. Other predisposing risk factors for meningitis associated with gram-negative bacilli include the following:
- Neurosurgical procedures or intracranial manipulation
- Old age
- Immunosuppression
- High-grade gram-negative bacillary bacteremia
- Disseminated strongyloidiasis
Disseminated strongyloidiasis has been reported as a classic cause of gram-negative bacillary bacteremia, as a result of the translocation of gut microflora with the Strongyloides stercoralis larvae during hyperinfection syndrome.
Staphylococcal meningitis
Staphylococci are gram-positive cocci that are part of the normal skin flora. Meningitis caused by staphylococci is associated with the following risk factors:
- Neurosurgery
- Head trauma
- Presence of CSF shunts
- Infective endocarditis and paraspinal infection
Epidemiology
The incidence of meningitis varies according to the specific etiologic agent, as well as in conjunction with a nation’s medical resources. The incidence is presumed to be higher in developing countries because of less access to preventive services, such as vaccination. In these countries, the incidence has been reported to be 10 times higher than that in developed countries.
Meningitis affects people of all races. In the United States, black people have a higher reported rate of meningitis than white people and Hispanic people.
Epidemiology of bacterial meningitis
With almost 4100 cases and 500 deaths occurring annually in the United States, bacterial meningitis continues to be a significant source of morbidity and mortality. The annual incidence in the United States is 1.33 cases per 100,000 population.[5]
Meningococcal meningitis is endemic in parts of Africa, India, and other developing areas. Periodic epidemics occur in the so-called sub-Saharan “meningitis belt,” as well as among religious pilgrims traveling to Saudi Arabia for the Hajj. In parts of Africa, widespread epidemics of meningococcal meningitis occur regularly. In 1996, the biggest wave of meningococcal meningitis outbreaks ever recorded arose in West Africa. An estimated 250,000 cases and 25,000 deaths occurred in Niger, Nigeria, Burkina Faso, Chad, and Mali.
The incidence of neonatal bacterial meningitis is 0.25-1 case per 1000 live births. In addition, the incidence is 0.15 case per 1000 full-term births and 2.5 cases per 1000 premature births. Approximately 30% of newborns with clinical sepsis have associated bacterial meningitis.
N meningitidis causes approximately 4 cases per 100,000 children aged 1-23 months. The risk of secondary meningitis is 1% for family contacts and 0.1% for daycare contacts. The rate of meningitis caused by S pneumoniae is 6.5 cases per 100,000 children aged 1-23 months.
Previously, Hib, N meningitidis, and S pneumoniae accounted for more than 80% of cases of bacterial meningitis. Since the late 20th century, however, the epidemiology of bacterial meningitis has been substantially changed by multiple developments.
The overall incidence of bacterial meningitis in the US declined from 2.0 to 1.38 cases per 100,000 population between 1998 and 2007.[5] This was partially because of the widespread use of the Hib vaccination, which decreased the incidence of H influenzae meningitis by more than 90% (see Table 3 below). Routine Hib vaccination has nearly eliminating this pathogen as a cause of meningitis in many developed countries.
More recent prevention measures such as the pneumococcal conjugate vaccine and universal screening of pregnant women for GBS have further changed the epidemiology of bacterial meningitis.
Table 3. Changing Epidemiology of Acute Bacterial Meningitis in United States*
| Bacteria | 1978-1981 | 1986 | 1995 | 1998-2007 | |
| Haemophilus influenzae | 48% | 45% | 7% | 6.7% | |
| Listeria monocytogenes | 2% | 3% | 8% | 3.4% | |
| Neisseria meningitidis | 20% | 14% | 25% | 13.9% | |
| Streptococcus agalactiae (group B streptococcus) | 3% | 6% | 12% | 18.1% | |
| Streptococcus pneumoniae | 13% | 18% | 47% | 58% | |
| *Nosocomial meningitis is not included; these data include only the 5 major meningeal pathogens. | |||||
The number of cases of invasive H influenzae disease among children younger than 5 years that were reported to the CDC declined from 20,000 in 1987 to 255 in 1998. This shift has reportedly been less dramatic in developing countries, where the use of Hib vaccine is not as widespread.
Because the frequency of bacterial meningitis in children has declined, the condition is becoming more of a disease of adults. Whereas the median age for persons with bacterial meningitis was 25 years in 1998, it was 15 months in 1986.
The introduction of vaccines against S pneumoniae has substantially reduced the incidence of pneumococcal meningitis in children. Routine screening for GBS in pregnant women may have also reduced the incidence of meningitis from this pathogen . Routine vaccination against serogroup C meningococcus may also reduce the incidence of N meningitidis infections. During a 1998-2007 survey, the incidence of meningitis declined by 31%, a decrease that can be credited to vaccination programs.
Newborns are at highest risk for acute bacterial meningitis. After the first month of life, the peak incidence is in infants aged 3-8 months. In addition, the incidence is increased in persons aged 60 years and older, independent of other factors. The annual incidence ranges from 1.7 to 7.2 cases per 100,000 adults; the mean annual incidence has been reported as 3.8 cases per 100,000 adults. Of patients with bacterial meningitis, 61% had no previous or present accompanying diseases that may have predisposed them to meningitis.
Depending on their age, individuals are also predisposed to meningitis from other etiologic agents (see Table 4 below). E coli K1 meningitis and S agalactiaemeningitis are common among neonates, and L monocytogenes meningitis is common among neonates and the elderly. (The development of neonatal meningitis is related to labor and delivery; it results from colonized pathogens in the maternal intestinal or genital tract, immaturity, and environment.)
Epidemiology of specific bacterial pathogens of acute meningitis
H influenzae meningitis primarily affects infants younger than 2 years. S agalactiaemeningitis occurs principally during the first 12 weeks of life but has also been reported in adults, primarily affecting individuals older than age 60 years. The overall case-fatality rate in adults is 34%. Among the bacterial agents that cause meningitis, S pneumoniae is associated with one of the highest mortalities (19-26%).
Epidemiology of aseptic meningitis
Aseptic meningitis has a reported incidence of 10.9 cases per 100,000 person-years. It occurs in individuals of all ages but is more common in children, especially during summer. No racial differences are reported. Aseptic meningitis tends to occur 3 times more frequently in males than in females.
Viruses are the major cause of aseptic meningitis. The enteroviruses are distributed worldwide, and the infection rates vary according to the season of the year and a population’s age and socioeconomic status. Most enteroviral infections occur in individuals who are younger than 15 years, with the highest attack rates in children who are younger than 1 year.
Arboviruses are an important cause of aseptic meningitis and encephalitis in the summer and fall months in the United States. West Nile virus was introduced to the United States in 1999 and has now spread throughout the continent. In 2012, the largest outbreak of West Nile virus infection to date occurred in the United States, with 5387 cases reported (about half of which were neuroinvasive disease, such as meningitis or encephalitis) and a 4.5% mortality. West Nile virus can also cause acute flaccid paralysis, retinitis and nephropathy.
Other less common arboviruses include St Louis encephalitis virus, Jamestown canyon virus, La Crosse encephalitis virus, Powassan encephalitis virus, and Eastern equine encephalitis virus. In the United States, the last epidemic of St Louis encephalitis was in Monroe, Louisiana, in 2001; 63 cases were reported, with 3 deaths (4.7% mortality). Infection with the La Crosse encephalitis virus also usually occurs during the summer and early fall, with symptoms again being typical of acute aseptic meningitis.
Infections with the LCM virus occur worldwide. Most human cases occur among young adults during autumn.
Of fungal causes, B dermatitidis is reportedly endemic in North America (eg, Mississippi and Ohio River basins). It has also been isolated from parts of Central America, South America, the Middle East, and India. H capsulatum has been reported from many areas of the world, with the Mississippi and Ohio River valleys being the most endemic regions in North America.
Of parasitic causes, A cantonensis is common in Southeast Asia and the Pacific Islands. It has also been found in rats outside this region, particularly in regions of Africa, Puerto Rico, and Louisiana, presumably introduced by ship-borne rats from endemic areas. G spinigerum is common in Southeast Asia, China, and Japan but has been reported sporadically worldwide.
Epidemiology of chronic meningitis
Brucella -associated chronic meningitis has a worldwide distribution and is common in the Middle East, India, Mexico, and Central and South America. In the United States, after the control of bovine infections, the incidence decreased to less than 0.5 cases per 100,000 population, and only 79 cases were reported to the CDC in 1998.
M tuberculosis is worldwide in distribution, and humans are its only reservoir. In 1997, the estimated case rates among endemic countries ranged from 62 to 411 cases per 100,000 population.
B burgdorferi is a tick-borne spirochete that is found in the temperate regions of much of the northern hemisphere. Endemic regions include North America (eg, the northeastern United States, Minnesota, Wisconsin, and parts of California and Oregon), Europe, and Asia.
C neoformans has a worldwide distribution. Serotypes B and C have been restricted mostly to tropical and subtropical regions, and serotype B has been isolated from eucalyptus trees.
The distribution of C immitis is limited to the endemic regions of the Western Hemisphere, within the north and south 40° latitudes (ie, parts of the southwestern United States, Mexico, and Central and South America). Persons who have migrated from or traveled to endemic areas may experience onset of disease in other parts of the world.
S schenckii has been reported worldwide. However, most cases come from the tropical regions of the Americas.
Prognosis
Patients with meningitis who present with an impaired level of consciousness are at increased risk for neurologic sequelae or death. A seizure during an episode of meningitis also is a risk factor for mortality or neurologic sequelae, particularly if the seizure is prolonged or difficult to control.
In bacterial meningitis, several risk factors are associated with death and with neurologic disability. A risk score has been derived and validated in adults with bacterial meningitis. This score includes the following variables, which are associated with an adverse clinical outcome :
- Older age
- Increased heart rate
- Lower Glasgow Coma Scale score
- Cranial nerve palsies
- CSF leukocyte count lower than 1000/μL
- Gram-positive cocci on CSF Gram stain
Advanced bacterial meningitis can lead to brain damage, coma, and death. In 50% of patients, several complications may develop in the days to weeks following infection. Long-term sequelae are seen in as many as 30% of survivors and vary with etiologic agent, patient age, presenting features, and hospital course. Patients usually have subtle CNS changes.
Serious complications include the following:
- Hearing loss
- Cortical blindness
- Other cranial nerve dysfunction
- Paralysis
- Muscular hypertonia
- Ataxia
- Multiple seizures
- Mental motor retardation
- Focal paralysis
- Ataxia
- Subdural effusions
- Hydrocephalus
- Cerebral atrophy
Risk factors for hearing loss after pneumococcal meningitis are female gender, older age, severe meningitis, and infection with certain pneumococcal serotypes (eg, 12F). Delayed complications include the following:
- Decreased hearing or deafness
- Other cranial nerve dysfunctions
- Multiple seizures
- Focal paralysis
- Subdural effusions
- Hydrocephalus
- Intellectual deficits
- Ataxia
- Blindness
- Waterhouse-Friderichsen syndrome
- Peripheral gangrene
Seizures are a common and important complication, occurring in approximately one fifth of patients. The incidence is higher in patients younger than 1 year, reaching 40%. Approximately one half of patients with this complication have repeated seizures. Patients may die as a result of diffuse CNS ischemic injury or systemic complications.
Even with effective antimicrobial therapy, significant neurologic complications have been reported to occur in as many as 30% of survivors of bacterial meningitis. Close monitoring for the development of these complications is essential.
Mortality for bacterial meningitis is highest in the first year of life, decreases in midlife, and increases again in old age. Bacterial meningitis is fatal in 1 in 10 cases, and 1 of every 7 survivors is left with a severe handicap, such as deafness or brain injury.
The prognosis in patients with meningitis caused by opportunistic pathogens depends on the underlying immune function of the host. Many patients who survive the disease require lifelong suppressive therapy (eg, long-term fluconazole for suppression in patients with HIV-associated cryptococcal meningitis).
Among bacterial pathogens, S pneumoniae causes the highest mortality (20-30% in adults, 10% in children) and morbidity (15%) in meningitis. If severe neurologic impairment is evident at the time of presentation (or if the onset of illness is extremely rapid), mortality is 50-90% and morbidity is even higher, even with immediate medical treatment. Meningitis caused by L monocytogenes or gram-negative bacilli also has a higher case-fatality rate than meningitis caused by other bacterial agents.
Reported overall mortality for meningitis from specific bacterial organisms is as follows:
- S pneumoniae - 19-26%
- H influenzae - 3-6%
- N meningitidis - 3-13%
- L monocytogenes - 15-29%
Patients with meningococcal meningitis have a better prognosis than do those with pneumococcal meningitis, with a mortality of 4-5%; however, patients with meningococcemia have a poor prognosis, with a mortality of 20-30%.
The mortality for viral meningitis without encephalitis is less than 1%. In patients with deficient humoral immunity (eg, agammaglobulinemia), enteroviral meningitis may have a fatal outcome. Patients with viral meningitis usually have a good prognosis for recovery. The prognosis is worse for patients at the extremes of age (ie, < 2 or >60 years) and those with significant comorbidities and underlying immunodeficiency.
History
Only about 44% of adults with bacterial meningitis exhibit the classic triad of fever, headache, and neck stiffness. These symptoms can develop over several hours or over 1-2 days. In a large prospective study of 696 cases of adults with bacterial meningitis, van de Beek et al reported that 95% of the patients had 2 out of the following 4 symptoms: fever, headache, stiff neck, and altered mental status.
Other symptoms can include the following:
- Nausea
- Vomiting
- Photalgia (photophobia) - Discomfort when the patient looks into bright lights
- Sleepiness
- Confusion
- Irritability
- Delirium
- Coma
Approximately 25% of patients with bacterial meningitis present acutely, well within 24 hours of the onset of symptoms. Occasionally, if a patient has been taking antibiotics for another infection, meningitis symptoms may take longer to develop or may be less intense.
Approximately 25% of patients have concomitant sinusitis or otitis that could predispose to S pneumoniae meningitis.[12] In contrast, patients with subacute bacterial meningitis and most patients with viral meningitis present with neurologic symptoms developing over 1-7 days. Chronic symptoms lasting longer than 1 week suggest the presence of meningitis caused by certain viruses or by tuberculosis, syphilis, fungi (especially cryptococci), or carcinomatosis.
Patients with viral meningitis may have a history of preceding systemic symptoms (eg, myalgias, fatigue, or anorexia). Patients with meningitis caused by the mumps virus usually present with the triad of fever, vomiting, and headache. This follows the onset of parotitis (salivary gland enlargement occurs in 50% of patients), which clinically resolves in 7-10 days.
As bacterial meningitis progresses, patients of any age may have seizures (30% of adults and children; 40% of newborns and infants). In patients who have previously been treated with oral antibiotics, seizures may be the sole presenting symptom; fever and changes in level of alertness or mental status are less common in partially treated meningitis than in untreated meningitis.
Atypical presentation may be observed in certain groups. Elderly individuals, especially those with underlying comorbidities (eg, diabetes, renal and liver disease), may present with lethargy and an absence of meningeal symptoms. Patients with neutropenia may present with subtle symptoms of meningeal irritation.
Other immunocompromised hosts, including organ and tissue transplant recipients and patients with HIV and AIDS, may also have an atypical presentation. Immunosuppressed patients may not show dramatic signs of fever or meningeal inflammation.
A less dramatic presentation―headache, nausea, minimal fever, and malaise―may be found in patients with low-grade ventriculitis associated with a ventriculoperitoneal shunt. Newborns and small infants also may not present with the classic symptoms, or the symptoms may be difficult to detect. An infant may appear only to be slow or inactive, or be irritable, vomiting, or feeding poorly. Other symptoms in this age group include temperature instability, high-pitched crying, respiratory distress, and bulging fontanelles (a late sign in one third of neonates).
Epidemiologic factors and predisposing risks should be assessed in detail. These may suggest the specific etiologic agent.
Exposures
A history of exposure to a patient with a similar illness is an important diagnostic clue. It may point to the presence of epidemic disease, such as viral or meningococcal meningitis.
Elicit any history of sexual contact or high-risk behavior from the patient. Herpes simplex virus (HSV) meningitis is associated with primary genital HSV infection and HIV infection. A history of recurrent bouts of benign aseptic meningitis suggests Mollaret syndrome, which is caused by HSV.
Animal contacts should be elicited. Patients with rabies could present atypically with aseptic meningitis; rabies should be suspected in a patient with a history of animal bite (eg, from a skunk, raccoon, dog, fox, or bat). Exposure to rodents suggests infection with lymphocytic choriomeningitis virus (LCM) virus andLeptospira infection. Laboratory workers dealing with these animals also are at increased risk of contracting LCM.
Brucellosis may be transmitted through contact with infected farm animals (eg, cows or pigs). The intake of unpasteurized milk and cheese also predisposes to brucellosis, as well as to L monocytogenes infection.
Previous medical treatment and existing conditions
A history of recent antibiotic use should be elicited. As many as 40% of patients who present with acute or subacute bacterial meningitis have previously been treated with oral antibiotics (presumably because of misdiagnosis at the time of initial presentation).
The presence of a ventriculoperitoneal shunt or a history of recent cranial surgery should be elicited. Patients with low-grade ventriculitis associated with a ventriculoperitoneal shunt may have a less dramatic presentation than those with acute bacterial meningitis, experiencing headache, nausea, minimal fever, and malaise. The presence of cochlear implants with a positioner has been associated with a higher risk of bacterial meningitis.
Alcoholism and cirrhosis are risk factors for meningitis. Unfortunately, the multiple etiologies of fever and seizures in patients with alcoholism or cirrhosis make meningitis challenging to diagnose.
Location and travel
Geographic location and travel history are important in the evaluation of patients. Infection with H capsulatum or B dermatitidis is considered in patients with exposure to endemic areas of the Mississippi and Ohio River valleys; C immitis is considered in regions of the southwestern United States, Mexico, and Central America. B burgdorferi is considered in regions of the northeastern and northern central United States, if tick exposure is a possibility.
Season and temperature
The time of year is an important variable because many infections are seasonal. With enteroviruses (which are found worldwide), infections occur during late summer and early fall in temperate climates and year-round in tropical regions. In contrast, mumps, measles, and varicella-zoster virus (VZV) are more common during winter and spring. Arthropod-borne viruses (eg, West Nile virus, St Louis encephalitis, and California encephalitis virus) are more common during the warmer months.
Physical Examination
The classic triad of meningitis consists of fever, nuchal rigidity, and altered mental status, but not all patients have all 3, and almost all patients have headache. Altered mental status can range from irritability to somnolence, delirium, and coma. The examination reveals no focal neurologic deficits in the majority of cases. Furthermore, the majority of patients with bacterial meningitis have a stiff neck, but the meningeal signs are insensitive for diagnosis of meningitis.
Acute bacterial meningitis in otherwise healthy patients who are not at the extremes of age presents in a clinically obvious fashion. In contrast, most patients with subacute bacterial meningitis pose a diagnostic challenge. Systemic examination occasionally reveals a pulmonary or otitis media coinfection.
Systemic findings can also be present. Extracranial infection (eg, sinusitis, otitis media, mastoiditis, pneumonia, or urinary tract infection [UTI]) may be noted. Endotoxic shock with vascular collapse is characteristic of severe N meningitidis(meningococcal) infection.
General physical findings in viral meningitis are common to all causative agents, but some viruses produce unique clinical manifestations that help focus the diagnostic approach. Enteroviral infection is suggested by the presence of the following:
- Exanthemas
- Symptoms of pericarditis, myocarditis, or conjunctivitis
- Syndromes of pleurodynia, herpangina, and hand-foot-and-mouth disease
Increased blood pressure with bradycardia can also be present. Vomiting occurs in 35% of patients.
Nonblanching petechiae and cutaneous hemorrhages may be present in meningitis caused by N meningitidis (50%), H influenzae, S pneumoniae, or S aureus. Arthritis is seen with meningococcal infection and with M pneumoniae infection but is less common with other bacterial species.
Infants
Infants may have the following:
- Bulging fontanelle (if euvolemic)
- Paradoxic irritability (ie, remaining quiet when stationary and crying when held)
- High-pitched cry
- Hypotonia
In infants, the clinicians should examine the skin over the entire spine for dimples, sinuses, nevi, or tufts of hair. These may indicate a congenital anomaly communicating with the subarachnoid space.
Focal neurologic signs
Focal neurologic signs include isolated cranial nerve abnormalities (principally of cranial nerves III, IV, VI, and VII), which are present in 10-20% of patients. These result from increased intracranial pressure (ICP) or the presence of exudates encasing the nerve roots. Focal cerebral signs are present in 10-20% of patients and may develop as a result of ischemia from vascular inflammation and thrombosis.
Papilledema is a rare finding (< 1% of patients) that also indicates increased ICP, but it is neither sensitive nor specific: it occurs in only one third of meningitis patients with increased ICP and is present not only in meningitis but also in brain abscess and other disorders.
Signs of meningeal irritation
For more than 100 years, clinicians have relied on meningeal signs (nuchal rigidity, Kernig sign, and Brudzinski sign) to evaluate patients with suspected meningitis and help determine who should undergo a lumbar puncture (LP). However, a prospective study of 297 adults with suspected meningitis documented very low sensitivities for these signs: 5% for the Kernig sign, 5% for the Brudzinski sign, and 30% for nuchal rigidity.[13] Thus, the absence of the meningeal signs should not defer the performance of the LP.
Systemic and extracranial findings
Systemic findings on physical examination may provide clues to the etiology of a patient’s meningitis. Morbilliform rash with pharyngitis and adenopathy may suggest a viral etiology (eg, Epstein-Barr virus [EBV], cytomegalovirus [CMV], adenovirus, or HIV). Macules and petechiae that rapidly evolve into purpura suggest meningococcemia (with or without meningitis). Vesicular lesions in a dermatomal distribution suggest VZV. Genital vesicles suggest HSV-2 meningitis.
Sinusitis or otitis suggests direct extension into the meninges, usually with S pneumoniae or, less often, H influenzae. Rhinorrhea or otorrhea suggests a cerebrospinal fluid (CSF) leak from a basilar skull fracture, with meningitis most commonly caused by S pneumoniae.
Hepatosplenomegaly and lymphadenopathy suggest a systemic disease, including viral (eg, mononucleosislike syndrome in EBV, CMV, and HIV) and fungal (eg, disseminated histoplasmosis). The presence of a heart murmur suggests infective endocarditis with secondary bacterial seeding of the meninges.
Chronic meningitis
It is essential to perform careful general, systemic, and neurologic examinations, looking especially for the following:
- Lymphadenopathy
- Papilledema and tuberculomas during funduscopy
- Meningismus
- Cranial nerve palsies
Tuberculous meningitis
The presentation of chronic tuberculous meningitis may be acute, but the classic presentation is subacute and spans weeks. Patients generally have a prodrome consisting of fever of varying degrees, malaise, and intermittent headaches. Cranial nerve palsies (III, IV, V, VI, and VII) often develop, suggesting basilar meningeal involvement.
Clinical staging of tuberculous meningitis is based on neurologic status, as follows:
- Stage 1 - No change in mental function, with no deficits and no hydrocephalus
- Stage 2 - Confusion and evidence of neurologic deficit
- Stage 3 - Stupor and lethargy
Syphilitic meningitis
The median incubation period before the appearance of symptoms in chronic syphilitic meningitis is 21 days (range, 3-90 days), during which time spirochetemia develops. Syphilitic meningitis usually occurs during the primary or secondary stage of syphilis, complicating 0.3-2.4% of primary infections during the first 2 years. Its presentation is similar to those of other types of aseptic meningitis, including headache, nausea, vomiting, and meningismus.
Meningovascular syphilis occurs later in the course of untreated syphilis, and the symptoms are dominated by focal syphilitic arteritis (ie, focal neurologic symptoms associated with signs of meningeal irritation) that spans weeks to months and results in stroke and irreversible damage if left untreated. Patients with concomitant HIV infection have an increased risk of accelerated progression.
Lyme meningitis
Although rare during stage 1 of Lyme disease, central nervous system (CNS) involvement with meningitis may occur in Lyme disease–associated chronic meningitis and is characterized by the concurrent appearance of erythema migrans at the site of the tick bite. More commonly, aseptic meningitis syndrome occurs 2-10 weeks after the erythema migrans rash. This represents stage 2 of Lyme disease, or the borrelial hematogenous dissemination stage.
Headache is the most common symptom of Lyme disease–associated chronic meningitis, with photophobia, nausea, and neck stiffness occurring less frequently. Somnolence, emotional lability, and impaired memory and concentration may occur. Facial nerve palsy is the most common cranial nerve deficit. These symptoms of meningitis usually fluctuate and may last for months if left untreated.
Fungal meningitis
Meningitis from C neoformans usually develops in patients with defective cell-mediated immunity (see CNS Cryptococcosis in HIV). It is characterized by the gradual onset of symptoms, the most common of which is headache.
Coccidioidal meningitis is the most serious form of disseminatedcoccidioidomycosis; it usually is fatal if left untreated. These patients may present with headache, vomiting, and altered mental function associated with pleocytosis, elevated protein levels, and decreased glucose levels. Eosinophils may be a prominent finding on CSF analysis.
Patients infected with B dermatitidis may present with an abscess or fulminant meningitis. Patients infected with H capsulatum may present with headache, cranial nerve deficits, or changes in mental status months before diagnosis.
Helminthic eosinophilic meningitis
After ingestion of A cantonensis larvae, which are found in raw or undercooked mollusks, most patients with symptomatic disease present with nonspecific and self-limited abdominal pain caused by larval migration into the bowel wall. On rare occasions, the larvae can migrate into the CNS and cause eosinophilic meningitis. Although A cantonensis is prevalent in Southeast Asia and tropical Pacific islands, infestations from this parasitic nematode have been reported in the United States and the Caribbean.[15]
Aseptic meningitis
In contrast to patients with bacterial meningitis, patients with aseptic meningitis syndrome usually appear clinically nontoxic, with no vascular instability. (SeeAseptic Meningitis.) In many cases, a cause for meningitis is not apparent after initial evaluation, and the condition is therefore classified as aseptic meningitis. These patients characteristically have an acute onset of meningeal symptoms, fever, and CSF pleocytosis that is usually prominently lymphocytic.
Complications
Immediate complications of meningitis include the following:
- Septic shock, including disseminated intravascular coagulation (DIC)
- Coma with loss of protective airway reflexes
- Seizures, which occur in 30-40% of children and 20-30% of adults
- Cerebral edema
- Septic arthritis
- Pericardial effusion
- Hemolytic anemia ( H influenzae)
Delayed complications include the following:
- Decreased hearing or deafness
- Other cranial nerve dysfunctions
- Multiple seizures
- Focal paralysis
- Subdural effusions
- Hydrocephalus
- Intellectual deficits
- Ataxia
- Blindness
- Waterhouse-Friderichsen syndrome
- Peripheral gangrene
Cerebral edema, cranial nerve palsy, and cerebral infarction
Some degree of cerebral edema is common with bacterial meningitis. This complication is an important cause of death.
Cranial nerve palsies and the effects of impaired cerebral blood flow, such as cerebral infarction, are caused by increased ICP. In certain cases, repeated LP or the insertion of a ventricular drain may be necessary to relieve the effects of this increase.
In cerebral infarction, endothelial cells swell, proliferate, and crowd into the lumen of the blood vessel, and inflammatory cells infiltrate the blood vessel wall. Foci of necrosis develop in the arterial and venous walls and induce arterial and venous thrombosis. Venous thrombosis is more frequent than arterial thrombosis, but arterial and venous cerebral infarctions can be seen in 30% of patients.
Brain parenchymal damage
Brain parenchymal damage is the most important and feared complication of bacterial meningitis. It can lead to the following disorders:
- Sensory and motor deficits
- Cerebral palsy
- Learning disabilities
- Mental retardation
- Cortical blindness
- Seizures
Cerebritis
Inflammation often extends along the perivascular (Virchow-Robin) spaces into the underlying brain parenchyma. Commonly, cerebritis results from direct spread of infection, either from otorhinologic infection or meningitis (including retrograde septic thrombophlebitis) or from hematogenous spread from an extracranial focus of infection. Parenchymal involvement, with edema and mass effect, may be localized or diffuse. Cerebritis can evolve to frank abscess formation in the gray matter–white matter junction.
Subdural effusion
In children with meningitis who are younger than 1 year, 20-50% of cases are complicated by sterile subdural effusions. Most of these effusions are transient and small to moderate in size. About 2% of them are infected secondarily and become subdural empyemas. In the empyema, infection and necrosis of the arachnoid membrane permit formation of a subdural collection.
In addition to young age, risk factors include rapid onset of illness, low peripheral white blood cell (WBC) count, and high CSF protein level. Seizures occur more commonly during the acute course of the disease, though long-term sequelae of promptly treated subdural effusions are similar to those of uncomplicated meningitis.
Ventriculitis
Ventriculitis may occur through the involvement of the ependymal lining of the ventricles. This complication occurs in 30% of patients overall but is especially common in neonates, with an incidence as high as 92%. The organisms enter the ventricles via the choroid plexuses. As a result of reduced CSF flow, and possibly of reduced secretion of CSF by the choroid plexus, the infective organisms remain in the ventricles and multiply.
Ventriculomegaly
Ventriculomegaly can occur early or late in the course of meningitis and is usually transient and mild to moderate in severity. As a result of the subarachnoid inflammatory exudate, CSF pathways may become obstructed, leading to hydrocephalus. Exudates in the foramina of Luschka and Magendie can cause noncommunicating hydrocephalus, whereas exudates that accumulate in the basilar cisterns or over the cerebral convexity can develop into communicating hydrocephalus.
Meningitis workup
Approach Considerations
The diagnostic challenges in patients with clinical findings of meningitis are as follows:
- Early identification and treatment of patients with acute bacterial meningitis
- Assessing whether a treatable central nervous system (CNS) infection is present in those with suspected subacute or chronic meningitis
- Identifying the causative organism
Bacterial meningitis must be the first and foremost consideration in the differential diagnosis of patients with headache, neck stiffness, fever, and altered mental status. Acute bacterial meningitis is a medical emergency, and delays in instituting effective antimicrobial therapy result in increased morbidity and mortality.
In general, whenever the diagnosis of meningitis is strongly considered, a lumbar puncture should be promptly performed. Examination of the cerebrospinal fluid (CSF) is the cornerstone of the diagnosis. The diagnosis of bacterial meningitis is made by culture of the CSF sample. The opening pressure should be measured and the fluid sent for cell count (and differential count), chemistry (ie, CSF glucose and protein), and microbiology (ie, Gram stain and cultures).
A concern regarding LP is that the lowering of CSF pressure from withdrawal of CSF could precipitate herniation of the brain. Herniation can sometimes occur in acute bacterial meningitis and other CNS infections as the consequence of severe cerebral edema or acute hydrocephalus. Clinically, this is manifested by an altered state of consciousness, abnormalities in pupil reflexes, and decerebrate or decorticate posturing. The incidence of herniation after LP, even in patients with papilledema, is approximately 1%.
A screening computed tomography (CT) scan of the head may be performed before LP to determine the risk of herniation. A prospective study involving 301 adults with suspected meningitis found that the following baseline patient characteristics were associated with an abnormal finding on head CT
- Age ≥60 years
- Immunocompromise (ie, HIV infection/AIDS, immunosuppressive therapy, or transplantation)
- A history of CNS disease
- A history of seizure within 1 week before presentation
- Any abnormality on neurologic examination
These factors have been included in the Infectious Diseases Society of America guidelines to decide who should undergo CT before LP.[17]
The decision to obtain a brain CT scan before LP should not delay the institution of antibiotic therapy; such delay can increase mortality. It should be also noted that herniation can occur in patients with bacterial meningitis who have a normal brain CT scan. The most reliable clinical signs that indicate the risk of herniation include deteriorating level of consciousness, brainstem signs, and a very recent seizure.
Other laboratory tests, which may include blood cultures, are needed to complement the CSF culture. These bacterial cultures are used for identification of the offending bacteria and occasionally its serogroup, as well as for determination of the organism’s susceptibility to antibiotics. Special studies, such as serology and nucleic acid amplification, may also be performed, depending on clinical suspicion of an offending organism.
As many as 50% of patients with pneumococcal meningitis also have evidence of pneumonia on initial chest radiography. This association occurs in fewer than 10% of patients with meningitis caused by H influenzae or N meningitidis and in approximately 20% of patients with meningitis caused by other organisms. (See Imaging in Bacterial Meningitis.)
Blood Studies
In patients with bacterial meningitis, a complete blood count (CBC) with differential will demonstrate polymorphonuclear leukocytosis with a left shift. Useful elements of the metabolic panel include the following:
- Serum electrolytes, to determine dehydration or syndrome of inappropriate secretion of antidiuretic hormone (SIADH)
- Serum glucose (which is compared with the CSF glucose)
- Blood urea nitrogen (BUN) or creatinine and liver profile, to assess organ function and adjust antibiotic dosing
The serum glucose level may be low if glycogen stores are depleted, or they may be high in infected patients with diabetes.
A coagulation profile and platelet count are indicated in cases of chronic alcohol use, chronic liver disease, or suspected disseminated intravascular coagulation (DIC). Patients with coagulopathies may require platelets or fresh frozen plasma (FFP) before LP.
Cultures and Bacterial Antigen Testing
Obtaining cultures before instituting antibiotics may be helpful if the diagnosis is uncertain. The utility of cultures is most evident when LP is delayed until head imaging can rule out the risk of brain herniation, in which cases antimicrobial therapy is rightfully initiated before CSF samples can be obtained. These cultures include the following:
- Blood - 50% positive in meningitis caused by H influenzae, S pneumoniae, orN meningitidis
- Nasopharynx
- Respiratory secretions
- Urine
- Skin lesions
Latex agglutination or counterimmunoelectrophoresis (CIE) of blood, urine, and CSF for specific bacterial antigens is occasionally recommended if diagnosis is challenging or in patients with partially treated meningitis. The Binax NOW S pneumoniae antigen test, if done on CSF, has a 99%-100% sensitivity and specificity and can even be positive despite prior antibiotic therapy.[18]
The use of nucleic acid amplification (eg, polymerase chain reaction [PCR] testing) has revolutionized the diagnosis of herpes simplex virus (HSV) meningitis. The availability of this technique has confirmed HSV as the cause of the recurrent Mollaret meningitis. This technique has also been applied to the diagnosis of enteroviral infections and the other herpesvirus infections. The PCR assay for enteroviruses has been demonstrated to be substantially more sensitive than culture and is 94-100% specific.
Syphilis Testing
Perform serologic tests to detect syphilis. Screening for syphilis is done with the nontreponemal tests: rapid plasma reagent (RPR) or Venereal Disease Research Laboratory (VDRL). Positive results are confirmed with one of the following specific treponemal tests:
- Fluorescent treponemal antibody absorption (FTA-Abs)
- T pallidum hemagglutination (TPHA)
- Microhemagglutination– T pallidum (MHA-TP)
- The newer immune-capture enzyme immunoassay (ICE Syphilis) recombinant antigen test
In patients with syphilis, initial results on nontreponemal tests can serve as a baseline for gauging the success of therapy. Titers decrease and usually revert to negative or undetectable levels following effective treatment.
Serum Procalcitonin Testing
Increasing data suggest that serum procalcitonin (PCT) levels can be used as a guide to distinguish between bacterial and aseptic meningitis in children. Elevated serum PCT levels predict bacterial meningitis. The results of serum PCT testing, combined with other findings, could be helpful in making clinical decisions.
In an analysis of retrospective, multicenter, hospital-based cohort studies, Dubos et al confirmed that measurement of the PCT level is the best biologic marker for differentiating bacterial meningitis from aseptic meningitis in children in the emergency department (ED). With a threshold of 0.5 ng/mL, the sensitivity and specificity of the PCT level in distinguishing between bacterial and aseptic meningitis were 99% and 83%, respectively.
Lumbar Puncture and CSF Analysis
Elevated opening pressure correlates with increased risk of morbidity and mortality in bacterial and fungal meningitis. In bacterial meningitis, elevated opening pressure (reference range, 80-200 mm H2 O) suggests increased intracranial pressure (ICP) from cerebral edema. In viral meningitis, the opening pressure is usually within the reference range. The CSF opening pressure may be elevated at times in cryptococcal meningitis, suggesting increased ICP, and it is usually elevated in tuberculous meningitis.
The CSF cell count varies according to the offending pathogen (see Tables 5 and 6 below). It is usually in the few hundreds (100-1000/µL) with a predominance of lymphocytes in patients with viral meningitis. Some cases of echovirus, mumps, and HSV meningitis may produce a neutrophilic picture early in the course of disease. (See Lumbar Puncture.)
Table 5. CSF Findings in Meningitis by Etiologic Agent (Open Table in a new window)
| Agent | Opening Pressure (mm H2 O) | WBC count (cells/µL) | Glucose (mg/dL) | Protein (mg/dL) | Microbiology |
| Bacterial meningitis | 200-300 | 100-5000; >80% PMNs | < 40 | >100 | Specific pathogen demonstrated in 60% of Gram stains and 80% of cultures |
| Viral meningitis | 90-200 | 10-300; lymphocytes | Normal, reduced in LCM and mumps | Normal but may be slightly elevated | Viral isolation, PCR assays |
| Tuberculous meningitis | 180-300 | 100-500; lymphocytes | Reduced, < 40 | Elevated, >100 | Acid-fast bacillus stain, culture, PCR |
| Cryptococcal meningitis | 180-300 | 10-200; lymphocytes | Reduced | 50-200 | India ink, cryptococcal antigen, culture |
| Aseptic meningitis | 90-200 | 10-300; lymphocytes | Normal | Normal but may be slightly elevated | Negative findings on workup |
| Normal values | 80-200 | 0-5; lymphocytes | 50-75 | 15-40 | Negative findings on workup |
| LCM = lymphocytic choriomeningitis; PCR = polymerase chain reaction; PMN = polymorphonuclear leukocyte; WBC = white blood cell. | |||||
Table 6. Comparison of CSF Findings by Type of Organism (Open Table in a new window)
| Normal Finding | Bacterial Meningitis | Viral Meningitis* | Fungal Meningitis** |
| Pressure (mm H2 O) 50-150 | Increased | Normal or mildly increased | Normal or mildly increased in tuberculous meningitis; may be increased in fungal; AIDS patients with cryptococcal meningitis have increased risk of blindness and death unless kept below 300 mm H2 O |
| Cell count (mononuclear cells/µL) Preterm: 0-25 Term: 0-22 >6 months: 0-5 | No cell count result can exclude bacterial meningitis; PMN count typically in 1000s but may be less dramatic or even normal (classically, in very early meningococcal meningitis and in extremely ill neonates); lymphocytosis with normal CSF chemistries seen in 15-25%, especially when cell counts < 1000 or with partial treatment; ~90% of patients with ventriculoperitoneal shunts who have CSF WBC count >100 are infected; CSF glucose is usually normal, and organisms are less pathogenic; cell count and chemistries normalize slowly (over days) with antibiotics | Cell count usually < 500, nearly 100% mononuclear; up to 48 hours, significant PMN pleocytosis may be indistinguishable from early bacterial meningitis; this is particularly true with eastern equine encephalitis; presence of nontraumatic RBCs in 80% of HSV meningoencephalitis, though 10% have normal CSF results | Hundreds of mononuclear cells |
| Microscopy No organisms | Gram stain 80% sensitive; inadequate decolorization may mistake Haemophilus influenzae for gram-positive cocci; pretreatment with antibiotics may affect stain uptake, causing gram-positive organisms to appear gram-negative and decrease culture yield by average of 20% | No organism | India ink is 50% sensitive for fungi; cryptococcal antigen is 95% sensitive; AFB stain is 40% sensitive for tuberculosis (increase yield by staining supernatant from at least 5 mL CSF) |
| Glucose Euglycemia: >50% serum Hyperglycemia: >30% serum Wait 4 hr after glucose load | Decreased | Normal | Sometimes decreased; aside from fulminant bacterial meningitis, lowest levels of CSF glucose are seen in tuberculous meningitis, primary amebic meningoencephalitis, and neurocysticercosis |
| Protein (mg/dL) Preterm: 65-150 Term: 20-170 >6 months: 15-45 | Usually >150, may be >1000 | Mildly increased | Increased; >1000 with relatively benign clinical presentation suggestive of fungal disease |
| AFB = acid-fast bacillus; CSF = cerebrospinal fluid; HSV = herpes simplex virus; RBC = red blood cell; PMN = polymorphonuclear leukocyte. *Some bacteria (eg, Mycoplasma, Listeria, Leptospira spp, Borrelia burgdorferi [Lyme], and spirochetes) produce spinal fluid alterations that resemble the viral profile. An aseptic profile also is typical of partially treated bacterial infections (>33% of patients have received antimicrobial treatment, especially children) and the 2 most common causes of encephalitis—the potentially curable HSV and arboviruses. **In contrast, tuberculous meningitis and parasites resemble the fungal profile more closely. | |||
CFS sample handling
After drawing the CSF sample, do the following with the tubes:
- Tube 1 – Send to the chemistry laboratory for glucose and protein
- Tube 2 – Send to the hematology laboratory for a cell count with differential
- Tube 3 – Send to the microbiology and immunology laboratory
- Tube 4 – Hold for a repeat cell count with differential, if needed (or for other subsequent studies not initially ordered)
Microbiology and immunology studies for tube 3 include the following:
- Gram stain
- Bacterial culture
- Acid-fast bacillus (AFB) stain and tuberculosis cultures
- India ink stain
- Cryptococcal antigen testing
- Fungal cultures, counterimmunoelectrophoresis ( CIE), VDRL, and cryptococcal antigen, if indicated
Tumor necrosis factor alpha (TNF-α), interleukin (IL)-1, and other cytokines have received increasing attention as mediators of the inflammatory response during bacterial meningitis. Leist et al reported detecting TNF-α in the CSF of 3 of 3 patients with bacterial meningitis, but in 0 of 7 patients with viral meningitis. Lopez-Cortez et al demonstrated that a TNF-α level higher than 150 pg/mL and an IL-1β level higher than 90 pg/mL showed sensitivities of 74% and 90%, respectively, in discriminating viral from aseptic meningitis.
Mustafa et al demonstrated that IL-1β can be detected in the CSF of 95% of infants and children with bacterial meningitis and that levels higher than 500 pg/mL were correlated with an increased risk of neurologic sequelae.[20]
These findings, though requiring both confirmation and amplification, suggest that analysis of TNF and other cytokines, in particular IL-1β, may prove valuable in differentiating acute bacterial meningitis from viral meningitis and possibly in detecting patients at particular risk for an adverse outcome. Their role in guiding adjunctive therapy, such as corticosteroids and nonsteroidal treatment of blood-brain barrier injury, is also under investigation.
CSF characteristics of acute bacterial meningitis
Examination of the CSF in patients with acute bacterial meningitis reveals the characteristic neutrophilic pleocytosis (cell count usually ranging from hundreds to a few thousand, with >80% PMNs). In some (25-30%) cases of L monocytogenesmeningitis, a lymphocytic predominance may occur. A low CSF white blood cell (WBC) count (< 20/µL) in the presence of a high bacterial load suggests a poor prognosis.
According to Seupaul, the following 3 findings on CSF analysis have clinically useful likelihood ratios for the diagnosis of bacterial meningitis in adults[21] :
- CSF glucose−to−blood glucose ratio of 0.4 or lower
- CSF WBC count of 500/µL or higher
- CSF lactate level of 31.53 mg/dL or higher
CSF characteristics of viral meningitis
In viral meningitis, the opening pressure is 90-200 mm H2 O, and the WBC count is 10-300/µL. Although the glucose concentration is typically normal, it can be below normal in meningitis from lymphocytic choriomeningitis virus (LCM), herpes simplex virus (HSV), mumps virus, and poliovirus. The protein concentration tends to be slightly elevated, but it can be within the reference range.
CSF characteristics of fungal meningitis
The diagnosis of cryptococcal meningitis relies on the identification of the pathogen in the CSF. The CSF is characterized by a lymphocytic pleocytosis (10-200/µL), a reduced glucose level, and an elevated protein level. The CSF picture of other fungal meningitides is similar to that of cryptococcal meningitis, usually with lymphocytic pleocytosis. Eosinophilic pleocytosis has rarely been associated with C immitis meningitis.
The definitive diagnosis usually relies on the demonstration of the specific fungal agent (eg, H capsulatum, C immitis, B dermatitidis, or Candida species) from clinical specimens, including the CSF. This could be in the form of fungal culture isolation (eg, C albicans growth from CSF).
More commonly, fungal serology (eg, presence of histoplasma antigen in the CSF) is used in the diagnosis of many cases of fungal meningitis because isolating these organisms from culture has proved difficult. It should be noted, however, that the serology for B dermatitidis is not accurate and a negative serology finding does not rule out the diagnosis.
A test used to detect fungal infection in the blood was successfully used in the diagnosis of fungal meningitis in an outbreak caused by contaminated steroids. This outbreak involved 13,534 US patients who underwent epidural steroid injection and were exposed to methylprednisolone acetate from lots contaminated with environmental fungi; hundreds of these individuals developed serious CNS complications. The test (Fungitell, Beacon Diagnostics Laboratories), which measures levels of b-D-glucan (a glycoprotein found in the fungal cell wall), was used in CSF samples from patients exposed to the contaminated steroids who had negative fungal culture and polymerase chain reaction results. All patients with fungal meningitis had detectable b-D-glucan in their CSF.
CSF characteristics of eosinophilic/parasitic meningitis
Primary amebic meningoencephalitis (PAM) caused by N fowleri is characterized by a neutrophilic pleocytosis, low glucose levels, elevated protein levels, and red blood cells (RBCs). Mononuclear pleocytosis may be observed in patients with subacute or chronic forms of PAM. Demonstration of the trophozoites, with the characteristic ameboid movement, on wet preparations of the CSF has been used for diagnosis. Alternatively, the ameba may be demonstrated in biopsy specimens.
In the presence of exposure, profound peripheral blood eosinophilia, and characteristic eosinophilic pleocytosis, suspicion of meningitis caused by A cantonensis, G spinigerum, or B procyonis should be entertained. Demonstrating the larvae ante mortem is usually difficult, and diagnosis relies on clinical presentation and a compatible epidemiologic history. Serologic tests may aid in the diagnosis. G spinigerum meningitis may mimic cerebrovascular disease in that it may cause cerebral hemorrhage.
CSF characteristics of Lyme meningitis
In patients with Lyme meningitis, the CSF is characterized by low-grade lymphocytic pleocytosis, low glucose levels, and elevated protein levels. Oligoclonal bands reactive to B burgdorferi antigens may be present. Demonstration of the specific antibody to B burgdorferi aids in the diagnosis.
Comparison between the antibody response in the CSF and that in the serum is a helpful diagnostic test. A CSF-to-serum ratio greater than 1 suggests intrathecal antibody production and neuroborreliosis.
CSF characteristics of tuberculous meningitis
In patients with tuberculous meningitis, the CSF is characterized by a predominantly lymphocytic pleocytosis; an elevated protein level, especially if a CSF block is present; and a low glucose level (< 40 mg/dL). PCR testing can provide a rapid diagnosis, though false-negative results may occur in samples containing very few organisms (< 2 colony-forming units [cfu]/mL). (SeeTuberculous Meningitis.)
CSF glucose and protein
In bacterial meningitis, the CSF glucose level (reference range, 40-70 mg/dL) is less than 40 mg/dL in 60% of patients. A simultaneous blood glucose determination should be obtained for the purposes of comparison. In patients with elevated blood glucose levels as a result of diabetes mellitus, the CSF-to-blood glucose ratio may not be predictive. The CSF glucose level is usually within the reference range in viral meningitis, but it may be low in some cases of LCM, HSV, mumps virus, or poliovirus infection.
The CSF protein level (reference range, 20-50 mg/dL) is usually elevated in bacterial meningitis. In viral meningitis, these levels are also usually elevated, though they can be within the reference range. In syphilitic meningitis, abnormal CSF protein levels (elevated) and CSF glucose levels (decreased) may be observed in 10-70% of cases.
CSF Gram stain and acid-fast bacillus stain
Gram staining of the CSF permits rapid identification of the bacterial cause in 60-90% of patients with bacterial meningitis. The presence of bacteria is 100% specific, but the sensitivity of this test for detection is variable. The likelihood of detection is higher in the presence of a higher bacterial concentration and diminishes with prior antibiotic use.
The demonstration of AFB (eg, with auramine-rhodamine stain, Ziehl-Neelsen stain, or Kinyoun stain) in the CSF is difficult and usually requires a large volume of CSF. Meningeal biopsy, with the demonstration of caseating granulomas and AFB on the smear, offers a higher yield than the CSF AFB smear.
CSF culture and antigen testing
CSF bacterial cultures yield the bacterial cause in 70-85% of cases. The yield diminishes by 20% in patients who have received antimicrobial therapy. In these cases, some experts advocate the use of a CSF bacterial antigen assay. This is a latex agglutination technique that can detect the antigens of H influenzae type B (Hib), S pneumoniae, N meningitidis, E coli K1, and S agalactiae (group B streptococcus [GBS]). Its theoretical advantage is the detection of the bacterial antigens even after microbial killing, as is observed after antibacterial therapy.
Another attractive alternative is using the Binax NOW for S pneumoniae in the CSF. This assay has a 99-100% sensitivity and specificity for ruling out the most common cause of bacterial meningitis.
Others studies, however, have shown that the CSF bacterial antigen assay may not be better than the Gram stain. Although it is specific (a positive result indicates a diagnosis of bacterial meningitis), a negative finding on the bacterial antigen test does not rule out meningitis (50-95% sensitivity).
Cryptococcal meningitis
C neoformans may be cultured from the CSF in cryptococcal meningitis. Other methods of identification include India ink preparation and the detection of CSF cryptococcal antigen. India ink has a sensitivity of only 50%, but it is highly diagnostic if positive.
Because of the low sensitivity of the India ink preparation, many centers have adapted the use of CSF cryptococcal antigen determination, a test with a sensitivity exceeding 90%. However, the CSF cryptococcal antigen determination is not universally available.
In instances when the India ink results are negative but the degree of clinical suspicion for cryptococcal meningitis is high, the CSF specimen may be sent to reference laboratories that can perform CSF cryptococcal antigen determination to confirm the diagnosis. In addition, the titer of the antigen could serve to monitor the response to treatment. Blood cultures and serum cryptococcal antigen should be obtained to determine whether cryptococcal fungemia is present.
Syphilitic meningitis
In syphilitic meningitis, isolating T pallidum from the CSF is extremely difficult and time-consuming. The spirochete could be demonstrated by using dark-field or phase-contrast microscopy on specimens collected from skin lesions (eg, chancres and other syphilitic lesions).
The diagnosis is usually supported by the CSF VDRL test, which has a sensitivity of 30-70% (a negative result on the CSF VDRL test does not rule out syphilitic meningitis) and a high specificity (a positive test result suggests the disease). Care must always be taken not to contaminate the CSF with blood during spinal fluid collection (eg, traumatic tap).
Lyme meningitis
CSF culture for B burgdorferi has a low yield. The CSF Lyme PCR assay, if available, offers a rapid, sensitive, and specific method of diagnosis. This assay is gaining popularity as the method of choice for diagnosing Lyme meningitis.
Cohn et al validated a clinical prediction rule for differentiating Lyme meningitis from aseptic meningitis. Their “rule of 7s” classifies children at low risk for Lyme meningitis when all of the following 3 criteria are met :
- < 7 days of headache
- < 70% CSF mononuclear cells
- Absence of cranial nerve VII palsy or other cranial nerve palsy
Tuberculous meningitis
Culture for Mycobacterium usually takes several weeks and may delay definitive diagnosis. M tuberculosis detection assays involving nucleic acid amplification have become available and have the advantages of rapidity, high sensitivity, and high specificity. There remains a need for mycobacterial growth in cultures because this method offers the advantage of performing drug susceptibility assays.
Viral isolation from CSF
Isolation of viruses from the CSF has a sensitivity of 65-70% for enteroviruses. Alternatively, isolation of enteroviruses from throat and stool viral cultures may also indirectly implicate enterovirus as the cause of the meningitis. Culture of mumps virus from the CSF has a low sensitivity (30-50%). LCM virus may be cultured in blood early in the disease or in urine at a later stage.
Neuroimaging
Computed tomography (CT) of the head and magnetic resonance imaging (MRI) of the brain generally do not aid in the diagnosis of meningitis. Some patients may show meningeal enhancement, but its absence does not rule out the condition. Routinely obtaining CT scans of the head may lead to unnecessary delay in the performance of diagnostic lumbar puncture and the initiation of antibiotic therapy; the latter may be detrimental to the outcome in these patients.
Cerebral herniation following the lumbar tap procedure is rare in individuals with no focal neurologic deficits and no evidence of increased ICP. If it occurs, it usually does so within 24 hours after the LP; thus, herniation should always be considered in the differential diagnosis if the patient’s neurologic status deteriorates during that time frame.
According to the Infectious Diseases Society of America guidelines, the following are indications for screening head CT before LP in adult patients :
- Immunocompromised state
- History of CNS disease (eg, mass lesion, stroke, or focal infection)
- Seizure within 1 week of presentation
- Papilledema
- Abnormal level of consciousness
- Focal neurologic deficit (eg, dilated nonreactive pupil, gaze palsy, or arm or leg drift)
In patients with suspected bacterial meningitis, blood cultures should be obtained and treatment initiated before imaging studies and LP. Neuroimaging may yield normal results or demonstrate small ventricles, effacement of sulci, and contrast enhancement over convexities (see the images below). Late findings include venous infarction and communicating hydrocephalus. Brain abscess, sinus or mastoid infection, skull fracture, and congenital anomalies must be ruled out. (See Imaging in Bacterial Meningitis.)
 Acute bacterial meningitis. This axial nonenhanced computed tomography scan shows mild ventriculomegaly and sulcal effacement.
Acute bacterial meningitis. This axial nonenhanced computed tomography scan shows mild ventriculomegaly and sulcal effacement. Acute bacterial meningitis. This axial T2-weighted magnetic resonance image shows only mild ventriculomegaly.
Acute bacterial meningitis. This axial T2-weighted magnetic resonance image shows only mild ventriculomegaly. Acute bacterial meningitis. This contrast-enhanced, axial T1-weighted magnetic resonance image shows leptomeningeal enhancement (arrows).
Acute bacterial meningitis. This contrast-enhanced, axial T1-weighted magnetic resonance image shows leptomeningeal enhancement (arrows).
Finally, neuroimaging studies are helpful in the detection of CNS complications of bacterial meningitis, such as the following (see the images below):
- Hydrocephalus
- Cerebral infarct
- Brain abscess
- Subdural empyema
- Venous sinus thrombosis
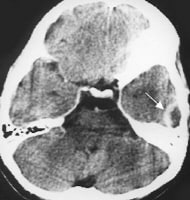 Chronic mastoiditis and epidural empyema in a patient with bacterial meningitis. This axial computed tomography scan shows sclerosis of the temporal bone (chronic mastoiditis), an adjacent epidural empyema with marked dural enhancement (arrow), and the absence of left mastoid air.
Chronic mastoiditis and epidural empyema in a patient with bacterial meningitis. This axial computed tomography scan shows sclerosis of the temporal bone (chronic mastoiditis), an adjacent epidural empyema with marked dural enhancement (arrow), and the absence of left mastoid air. Subdural empyema and arterial infarct in a patient with bacterial meningitis. This contrast-enhanced axial computed tomography scan shows left-sided parenchymal hypoattenuation in the middle cerebral artery territory, with marked herniation and a prominent subdural empyema.
Subdural empyema and arterial infarct in a patient with bacterial meningitis. This contrast-enhanced axial computed tomography scan shows left-sided parenchymal hypoattenuation in the middle cerebral artery territory, with marked herniation and a prominent subdural empyema.
Meningitis Treatment and Management
Approach Considerations
If the patient is in shock or hypotensive, crystalloid should be infused until euvolemia is achieved. If the patient’s mental status is altered, seizure precautions should be considered, seizures should be treated according to the usual protocol, and airway protection should be considered. If the patient is alert and in stable condition with normal vital signs, oxygen should be administered, intravenous (IV) access established, and rapid transport to the emergency department (ED) initiated. Institution of an ED triage protocol may help identify patients at risk.
In acute meningitis, regardless of presentation, a lumbar puncture (LP) and cerebrospinal fluid (CSF) examination are indicated to identify the causative organism and, in bacterial meningitis, the antibiotic sensitivities. Computed tomography (CT) of the head should be performed a before LP, if indicated. If no mass effect is present on head CT, LP is performed to obtain microbiology studies.
The performance of radiographic imaging should not delay the initiation of empiric antimicrobial therapy; such therapy should be initiated before head CT if indicated. It is vital to begin treatment as early as possible in the disease course; delay may contribute significantly to morbidity and mortality. In acutely ill patients, antibiotic therapy should be initiated promptly; in many of these cases, one should strongly consider giving adjunctive dexamethasone before the first antibiotic dose, or at least concomitantly with the dose.[17]
The patient’s condition and ED organization may warrant 8-12 hours of watchful waiting, followed by reexamination of the CSF (this should be done sooner if the patient’s condition deteriorates). If initial granulocytosis changes to mononuclear predominance, CSF glucose remains normal, and the patient continues to look well, the infection is most likely nonbacterial.
Treatment of complications
Systemic complications of acute bacterial meningitis must be treated, including the following:
- Hypotension or shock
- Hypoxemia
- Hyponatremia (from syndrome of inappropriate antidiuretic hormone secretion[SIADH])
- Cardiac arrhythmias and ischemia
- Stroke
- Exacerbation of chronic diseases
Signs of hydrocephalus and increasing intracranial pressure (ICP) should be watched for. Fever and pain should be managed, straining and coughing controlled, seizures prevented, and systemic hypotension avoided. In otherwise stable patients, sufficient care includes elevating the head and monitoring neurologic status. When more aggressive maneuvers are indicated, some authorities favor early use of diuresis (ie, furosemide 20 mg IV or mannitol 1 g/kg IV), provided that circulatory volume is protected.
Hyperventilation in intubated patients, with an arterial carbon dioxide tension (PaCO2) of 25-30 mm Hg as the goal, may briefly lower ICP; hyperventilation to a PaCO2 lower than 25 mm Hg may decrease cerebral blood flow disproportionately and lead to CNS ischemia. Placement of an ICP monitor should be considered in comatose patients or those with signs of increased ICP. With elevated ICP, CSF should be removed until pressure decreases by 50%; ICP should then be maintained at less than 300 mm H2 O.
Because seizure activity increases ICP, seizures must be aggressively controlled if present. Control may be accomplished by giving lorazepam 0.1 mg/kg IV and IV load with phenytoin 15 mg/kg or phenobarbital 5-10 mg/kg.
Treatment of Subacute Meningitis
In patients with subacute meningitis, CSF examination constitutes the critical step in documenting the presence or absence of a CNS infection and the type of infecting organism.
If the patient’s condition is serious and antibiotics have been given (potentially masking symptoms and hindering growth of organisms on culture), a bacterial infection is assumed to be present. Adequate antibiotic coverage is provided, and the patient is admitted. The LP is repeated if necessary to rule out partially treated bacterial meningitis.
Treatment of Bacterial Meningitis
Bacterial meningitis (including meningococcal meningitis, Haemophilus influenzaemeningitis, and staphylococcal meningitis) is a neurologic emergency that is associated with significant morbidity and mortality. Initiation of empiric antibacterial therapy is therefore essential for better outcome. (See tables 7 and 8 below.)
Table 7. Recommended Empiric Antibiotics for Suspected Bacterial Meningitis, According to Age or Predisposing Factors
| Age or Predisposing Feature | Antibiotics |
| Age 0-4 wk | Ampicillin plus either cefotaxime or an aminoglycoside |
| Age 1 mo-50 y | Vancomycin plus cefotaxime or ceftriaxone* |
| Age >50 y | Vancomycin plus ampicillin plus ceftriaxone or cefotaxime plus vancomycin* |
| Impaired cellular immunity | Vancomycin plus ampicillin plus either cefepime or meropenem |
| Recurrent meningitis | Vancomycin plus cefotaxime or ceftriaxone |
| Basilar skull fracture | Vancomycin plus cefotaxime or ceftriaxone |
| Head trauma, neurosurgery, or CSF shunt | Vancomycin plus ceftazidime, cefepime, or meropenem |
| CSF = cerebrospinal fluid. *Add ampicillin if Listeria monocytogenes is a suspected pathogen. | |
Table 8. Specific Antibiotics and Duration of Therapy for Acute Bacterial Meningitis
| Bacteria | Susceptibility | Antibiotic(s) | Duration (days) |
| Streptococcus pneumoniae | Penicillin MIC ≤0.06 μg/mL | Recommended: Penicillin G or ampicillin Alternatives: Cefotaxime, ceftriaxone, chloramphenicol | 10-14 |
| Penicillin MIC ≥0.12 μg/mL Cefotaxime or ceftriaxone MIC ≥0.12 μg/mL | Recommended: Cefotaxime or ceftriaxone Alternatives: Cefepime, meropenem | ||
| Cefotaxime or ceftriaxone MIC ≥1.0 μg/mL | Recommended: Vancomycin plus cefotaxime or ceftriaxone Alternatives: Vancomycin plus moxifloxacin | ||
| Haemophilus influenzae | Beta-lactamase−negative | Recommended: Ampicillin Alternatives: Cefotaxime, ceftriaxone, cefepime, chloramphenicol, aztreonam, a fluoroquinolone | 7 |
| Beta-lactamase−positive | Recommended: Cefotaxime or ceftriaxone Alternatives: Cefepime, chloramphenicol, aztreonam, a fluoroquinolone | ||
| Beta-lactamase−negative, ampicillin-resistant | Recommended: Meropenem Alternatives: Cefepime, chloramphenicol, aztreonam, a fluoroquinolone | ||
| Neisseria meningitidis | Penicillin MIC < 0.1 μg/mL | Recommended: Penicillin G or ampicillin Alternatives: Cefotaxime, ceftriaxone, chloramphenicol | 7 |
| Penicillin MIC ≥0.1 μg/mL | Recommended: Cefotaxime or ceftriaxone Alternatives: Cefepime, chloramphenicol, a fluoroquinolone, meropenem | ||
| Listeria monocytogenes | ... | Recommended: Ampicillin or penicillin G Alternative: TMP-SMX | 14-21 |
| Streptococcus agalactiae | ... | Recommended: Ampicillin or penicillin G Alternatives: Cefotaxime, ceftriaxone, vancomycin | 14-21 |
| Enterobacteriaceae | ... | Recommended: Cefotaxime or ceftriaxone Alternatives: Aztreonam, a fluoroquinolone, TMP-SMX, meropenem, ampicillin | 21 |
| Pseudomonas aeruginosa | ... | Recommended: Ceftazidime or cefepime Alternatives: Aztreonam, meropenem, ciprofloxacin | 21 |
| Staphylococcus epidermidis | Recommended: Vancomycin Alternative: Linezolid Consider addition of rifampin | ||
| MIC= minimal inhibitory concentration; TMP-SMX = trimethoprim-sulfamethoxazole. | |||
It is vital to institute empiric antimicrobial therapy (ie, antibacterial treatment or, in selected cases, antiviral or antifungal therapy) as soon as possible. The choice of agents is usually based on the known predisposing factors, initial CSF Gram stain results, or both. Once the pathogen has been identified and antimicrobial susceptibilities determined, the antibiotics may be modified for optimal targeted treatment.
Bacterial resistance, especially penicillin resistance among S pneumoniae strains, has been increasing worldwide. In March 2008, the US Food and Drug Administration (FDA) revised the susceptibility breakpoints for penicillin versus S pneumoniae. For nonmeningeal infections, the breakpoints are as follows:
- < 2 µg/mL – Susceptible
- 4 µg/mL – Intermediate
- >8 µg/mL – Resistant
For meningitis, the breakpoints are as follows:
- < 0.06 µg/mL – Susceptible
- ≥0.12 µg/mL – Resistant
With the new meningitis criteria (≥0.12 μg/mL), the prevalence of resistance was 34.8% in 2008, whereas with the old criteria (≥2 μg/mL), it was 12.3% for CSF. The geographic distribution of this resistance is variable, and it is important to know the regional patterns when deciding on local empiric antibiotic therapy (see Medication). A large observational study of 548 pneumococcal meningitis cases from Brazil showed that penicillin resistance was associated with higher mortality even after adjustment for age and severity of illness.
Appropriate antibiotic treatment for the most common types of bacterial meningitis reduces the risk of death. Mortality is higher with pneumococcal meningitis. In a nationwide observational cohort study from The Netherlands, adjunctive use of dexamethasone decreased pneumococcal meningitis mortality from 30% to 20%.
The chosen antibiotic should attain adequate levels in the CSF, and its ability to do so usually depends on its lipid solubility, molecular size, and protein-binding capacity, as well as on the patient’s degree of meningeal inflammation. The penicillins, certain cephalosporins (ie, third- and fourth-generation agents), the carbapenems, fluoroquinolones, and rifampin provide high CSF levels.
Monitoring for possible drug toxicity during treatment (eg, with blood counts and renal and liver function monitoring) is warranted. The antimicrobial dose must be adjusted on the basis of the patient’s renal and hepatic function. At times, obtaining serum drug concentrations may be necessary to ensure adequate levels and to avoid toxicity in drugs with a narrow therapeutic index (eg, vancomycin and aminoglycosides). The patient must also be monitored for complications from the disease (eg, hydrocephalus, seizures, or hearing defects).
Antibiotic therapy: neonates to age 1 month
In the first month of life, the most common microorganisms are group B or D streptococci, Enterobacteriaceae (eg, E coli), and L monocytogenes. Primary treatment consists of a combination of ampicillin and cefotaxime. The recommended dosage for cefotaxime is 50 mg/kg IV every 6 hours, up to 12 g/day. Ampicillin dosages are as follows:
- Age 0-7 days – 50 mg/kg IV every 8 hours
- Age 8-30 days – 50-100 mg/kg IV every 6 hours
Alternative treatment consists of ampicillin plus gentamicin. Gentamicin dosages are as follows:
- Age 0-7 days – 2.5 mg/kg IV or intramuscularly (IM) every 12 hours
- Age 8-30 days – 2.5 mg/kg IV or IM every 8 hours
Most authorities recommend adding acyclovir 10 mg/kg IV every 8 hours for herpes simplex encephalitis.
Antibiotic therapy: age 1-3 months
In infants 1-3 months of age, the first-line agent is cefotaxime (50 mg/kg IV every 6 hours, up to 12 g/day) or ceftriaxone (75 mg/kg initially, then 50 mg/kg every 12 hours, up to 4 g/day) plus ampicillin (50-100 mg/kg IV every 6 hours). An alternative agent is chloramphenicol (25 mg/kg orally or IV every 12 hours) plus gentamicin (2.5 mg/kg IV or IM every 8 hours).
If the local prevalence of drug-resistant S pneumoniae (DRSP) is higher than 2%, vancomycin (15 mg/kg IV every 8 hours) should be added. Treatment with dexamethasone (0.4 mg/kg IV every 12 hours for 2 days or 0.15 mg/kg IV every 6 hours for 4 days) should be strongly considered, starting 15-20 minutes before the first dose of antibiotics.
Antibiotic therapy: age 3 months to 7 years
In older infants or young children (age 3 months to 7 years), the most common microorganisms are S pneumoniae, N meningitidis, and H influenzae. Primary treatment is with either cefotaxime (50 mg/kg IV every 6 hours, up to 12 g/day) or ceftriaxone (75 mg/kg initially, then 50 mg/kg every 12 hours, up to 4 g/day).
If the prevalence of DRSP is greater than 2%, vancomycin (15 mg/kg IV every 8 hours) should be added. In countries with a low prevalence of DRSP, penicillin G (250,000 units/kg/day IM or IV in 3-4 divided doses) may be considered. Because of the increasing prevalence of DRSP, penicillin G is no longer recommended in the United States.
An alternative (which may also be chosen if the patient is severely allergic to penicillin) is chloramphenicol (25 mg/kg orally or IV every 12 hours) plus vancomycin (15 mg/kg IV every 8 hours). Treatment with dexamethasone (0.4 mg/kg IV every 12 hours for 2 days or 0.15 mg/kg IV every 6 hours for 4 days) should be strongly considered, starting 15-20 minutes before the first dose of antibiotics.
Antibiotic therapy: age 7-50 years
In an older child or an otherwise healthy adult (age 7-50 years), the most common microorganisms in bacterial meningitis are S pneumoniae, N meningitidis, and L monocytogenes. In areas where the prevalence of DRSP is greater than 2%, primary treatment consists of with either cefotaxime or ceftriaxone plus vancomycin. Pediatric dosing is as follows:
- Cefotaxime – 50 mg/kg IV every 6 hours, up to 12 g/day
- Ceftriaxone – 75 mg/kg initially, then 50 mg/kg every 12 hours, up to 4 g/day
- Vancomycin – 15 mg/kg IV every 8 hours
Adult dosing is as follows:
- Cefotaxime – 2 g IV every 4 hours
- Ceftriaxone – 2 g IV every 12 hours
- Vancomycin – 750-1000 mg IV every 12 hours or 10-15 mg/kg IV every 12 hours
Some experts add rifampin (pediatric dose, 20 mg/kg/day IV; adult dose, 600 mg/day orally). If Listeria is suspected, ampicillin (50 mg/kg IV every 6 hours) is added.
An alternative (which may also be chosen if the patient is severely penicillin-allergic) is chloramphenicol (12.5 mg/kg IV every 6 hours; not bactericidal) or clindamycin (pediatric dose, 40 mg/kg/day IV in 3-4 doses; adult dose, 900 mg IV every 8 hours; active in vitro but no clinical data) or meropenem (pediatric dose, 20-40 mg/kg IV every 8 hours; adult dose, 1 g IV every 8 hours; active in vitro but few clinical data). Imipenem is a proconvulsant and must be avoided.
In areas with a low prevalence of DRSP, cefotaxime or ceftriaxone plus ampicillin is recommended. Pediatric dosing is as follows:
- Cefotaxime – 50 mg/kg IV every 6 hours, up to 12 g/day
- Ceftriaxone – 75 mg/kg initially, then 50 mg/kg every 12 hours, up to 4 g/day
- Ampicillin – 50 mg/kg IV every 6 hours
Adult dosing is as follows:
- Cefotaxime – 2 g IV every 4 hours
- Ceftriaxone – 2 g IV every 12 hours
- Ampicillin – 50 mg/kg IV every 6 hours
An alternative (which may also be chosen if the patient is severely penicillin-allergic) is chloramphenicol (12.5 mg/kg IV every 6 hours) plus trimethoprim-sulfamethoxazole (TMP-SMX; TMP 5 mg/kg IV every 6 hours) or meropenem (pediatric dose, 20-40 mg/kg IV every 8 hours; adult dose, 1 g IV every 8 hours).
Data on the need for dexamethasone treatment in adults are limited, though there is support for its use in developed countries when S pneumoniae is the suspected organism. The first dose of dexamethasone (0.4 mg/kg every 12 hours IV for 2 days or 0.15 mg/kg every 6 hours for 4 days) should be administered 15-20 minutes before the first dose of antibiotics.
Antibiotic therapy: age ≥50 years
In adults older than 50 years or adults with disabling disease or alcoholism, the most common microorganisms are S pneumoniae, coliforms, H influenzae, Listeriaspecies, P aeruginosa, and N meningitidis.
Primary treatment, if the prevalence of DRSP is greater than 2%, is with either cefotaxime (2 g IV every 4 hours) or ceftriaxone (2 g IV every 12 hours) plus vancomycin (750-1000 mg IV every 12 hours or 10-15 mg/kg IV every 12 hours). If the CSF Gram stain shows gram-negative bacilli, ceftazidime (2 g IV every 8 hours) is given. In areas of low DRSP prevalence, treatment consists of cefotaxime (2 g IV every 4 hours) or ceftriaxone (2 g IV every 12 hours) plus ampicillin (50 mg/kg IV every 6 hours). Other options are meropenem, TMP-SMX, and doxycycline.
The Infectious Diseases Society of America guidelines recommend adjunctive dexamethasone in patients with suspected or proven community-acquired bacterial meningitis, but only in high-income countries. The first dose of dexamethasone (0.4 mg/kg IV every 12 hours for 2 days or 0.15 mg/kg every 6 hours for 4 days) is given 15-20 minutes before the first dose of antibiotics.
Dexamethasone should be continued if the culture grows either S pneumoniae or H influenzae. However, some experts advise that adjunctive treatment should be continued irrespective of the causative bacterium because of the low incidence of adverse events.
Antibiotic therapy: HIV-infected patients
In HIV-infected patients, if an ED workup does not identify a pathogen, serum and CSF samples should be drawn for cryptococcal antigen testing. Empiric treatment should proceed as in adults older than 50 years (pending results of all blood and CSF tests) to cover the bacterial pathogens, particularly S pneumoniae and L monocytogenes, for which this patient population is most at risk . (See Meningitis in HIV.)
Steroid therapy
The use of corticosteroids (typically, dexamethasone, 0.15 mg/kg every 6 hours for 2-4 days) as adjunctive treatment for bacterial meningitis improves outcome by attenuating the detrimental effects of host defenses (eg, inflammatory response to the bacterial products and the products of neutrophil activation). Controversy surrounds this practice, however, in that dexamethasone may interrupt the cytokine-mediated neurotoxic effects of bacteriolysis, which are at maximum in the first days of antibiotic use.
Theoretically, the anti-inflammatory effects of steroids decrease blood-brain barrier permeability and impede penetration of antibiotics into CSF. Decreased CSF levels of vancomycin have been confirmed in steroid-treated animals but not in comparably treated humans. Many authorities believe that all other antibiotics achieve minimal inhibitory concentrations (MICs) in CSF regardless of steroid use, and even vancomycin may not be affected to a clinically significant extent.
Nevertheless, the use of steroids has been shown to improve the overall outcome of patients with certain types of bacterial meningitis, including H influenzae,tuberculous, and pneumococcal meningitis.
In a meta-analysis by Brouwer et al, corticosteroids significantly reduced hearing loss and neurologic sequelae but did not reduce overall mortality. However, there was a trend toward lower mortality in adults receiving corticosteroids, and subgroup analyses showed that corticosteroids reduced severe hearing loss in H influenzaemeningitis and reduced mortality in S pneumoniae meningitis. However, the investigators found no beneficial effect for patients in low-income countries.
On the other hand, a meta-analysis of individual patient data by van de Beek et al was unable to identify which patients were most likely to benefit from dexamethasone treatment; indeed, no significant reduction in death or neurologic disability was found in any subgroups, including those determined by specific causative organisms, predexamethasone antibiotic treatment, HIV status, or age. The researchers concluded that the benefits of adjunctive dexamethasone in bacterial meningitis remain unproven.
In developing countries, the use of oral glycerol (rather than dexamethasone) has been studied as adjunctive therapy in the treatment of bacterial meningitis in children. In limited studies, it appears to reduce the incidence of neurologic sequelae while causing few side effects.
Intrathecal antibiotics
Intrathecal administration of antibiotics can be considered in patients with nosocomial meningitis (eg, meningitis developing after neurosurgery or placement of an external ventricular catheter) that does not respond to IV antibiotics. Although the FDA has not approved any antibiotics for intraventricular use, vancomycin and gentamicin are often used in this setting. Other agents used intrathecally include amikacin, polymyxin B, and colistin.
Intrathecal antibiotic dosages have been determined empirically and are adjusted on the basis of the CSF concentrations of the agent. Typical daily doses are as follows:
- Vancomycin: 5-20 mg
- Gentamicin: 1-2 mg in infants and children, 4–8 mg in adults
- Amikacin: 30 mg (range, 5-50 mg)
- Polymyxin B: 2 mg in infants and children, 5 mg in adults
- Colistin (usually formulated as colistimethate sodium): 10 mg once daily or 5 mg every 12 hours
Treatment of Viral Meningitis
Most cases of viral meningitis are benign and self-limited. Often, patients need only supportive care and require no specific therapy. In certain instances, specific antiviral therapy may be indicated, if available. Instituting antiretroviral therapy may be necessary for patients with HIV meningitis that occurs during an acute seroconversion syndrome. In patients with immune deficiency (eg, agammaglobulinemia), immunoglobulin replacement has been used to treat chronic enteroviral infections.
Herpes simplex meningitis
The antiviral management of HSV meningitis is controversial. Acyclovir (10 mg/kg IV every 8 hours) has been administered for HSV-1 and HSV-2 meningitis. Some experts do not advocate antiviral therapy unless associated encephalitis is present, because the condition is usually benign and self-limited. This is exemplified by Mollaret syndrome, a recurrent but benign syndrome of lymphocytic pleocytosis that is now attributed to HSV.
Cytomegalovirus meningitis
Ganciclovir and foscarnet are used for cytomegalovirus (CMV) meningitis in immunocompromised hosts. Ganciclovir is given in an induction dosage of 5 mg/kg IV every 12 hours for 21 days and a maintenance dosage of 5 mg/kg every 24 hours. Oral valganciclovir (900 mg/day) can be used for maintenance if immunosuppression continues (as, for example, in AIDS patients or transplant recipients). Foscarnet is given in an induction dosage of 60 mg/kg IV every 8 hours for 21 days and a maintenance dosage of 90-120 mg/kg IV every 24 hours.
Treatment of Fungal Meningitis
Causes of fungal meningitis include the following:
- Cryptococcus
- C immitis
- H capsulatum
- Candida species
- S schenckii (rarely)
Immune compromise is a predisposing factor in many of these cases and is often a consideration in the selection of treatment regimens.
Cryptococcal meningitis
Cryptococcal meningitis is a major opportunistic infection in AIDS patients. For initial therapy in these cases, administer amphotericin B (0.7-1 mg/kg/day IV) for at least 2 weeks, with or without flucytosine (100 mg/kg orally), in 4 divided doses. Liposomal preparations of amphotericin B may be used in patients who either have or are predisposed to develop renal dysfunction (amphotericin B liposome 3-4 mg/kg/day or amphotericin B lipid complex 5 mg/kg/day).
Fluconazole is given for consolidation therapy (400 mg/day for 8 weeks); itraconazole is an alternative if fluconazole is not tolerated. For maintenance therapy, long-term administration of fluconazole (200 mg/day) is most effective in preventing relapse (superior to itraconazole and amphotericin B at 1 mg/kg weekly). The risk of relapse is high in patients with AIDS.
In many cases, cryptococcal meningitis is complicated by increased ICP. Measuring the opening pressure during the LP is strongly advised. Efforts should be made to reduce such pressure through repetition of LP or insertion of a lumbar drain or a shunt. Medical maneuvers, such as administration of mannitol, have also been used.
The role of newer triazoles, such as voriconazole and posaconazole, has not been investigated. Echinocandins do not have activity against cryptococcus.
In resource-limited areas, amphotericin B and fluconazole are the optimal agents for treatment of HIV-related acute cryptococcal meningitis. Hence, treatment would consist of amphotericin and flucytosine, and policy makers and national departments of health in such countries should consider adding drugs that are typically unavailable in such settings (eg, flucytosine) for HIV treatment programs. (See Meningitis in HIV.)
Induction and consolidation therapy for cryptococcal meningitis in patients who do not have AIDS and are not transplant recipients involves giving amphotericin B (0.7-1 mg/kg/day) plus flucytosine (100 mg/kg/day) for at least 4 weeks. Treatment may be extended to 6 weeks in patients with neurologic complications. After this initial period, fluconazole (400 mg/day) is given for at least 8 weeks. LP is recommended after 2 weeks to document sterilization of the CSF. If the infection persists, longer induction therapy is recommended (6 weeks).
Solid-organ transplant recipients with cryptococcal meningitis should be treated with liposomal amphotericin B (3-4 mg/kg/day IV) or amphotericin B lipid complex (5 mg/kg/day IV) plus flucytosine (100 mg/kg/day in 4 divided doses) for at least 2 weeks of induction therapy. This is followed by consolidation treatment with fluconazole (400-800 mg/day orally for 8 weeks) and then maintenance treatment with fluconazole (200 mg/day orally for 6-12 months).
Coccidioides immitis
The preferred treatment for meningitis caused by C immitis is oral fluconazole (400 mg/day). Some physicians initiate therapy with a larger dose of fluconazole (as high as 1000 mg/day) or with a combination of fluconazole and intrathecal amphotericin B. Itraconazole (400-600 mg/day) has been reported to be comparably effective. Lifelong treatment is usually required. (See Coccidioidomycosis.)
Histoplasma capsulatum
The recommended treatment for H capsulatum meningitis is liposomal amphotericin B (5 mg/kg/day IV for a total of 175 mg/kg given over 4-6 weeks), followed by oral itraconazole (200-300 mg 2 or 3 times daily for at least 1 year or until the resolution of CSF abnormalities and Histoplasma antigen levels). Blood levels of itraconazole should be measured to ensure good absorption of the oral drug.
This infection is associated with a poor outcome. Approximately 20-40% of patients with meningitis succumb to the infection despite amphotericin B therapy, and 50% of responders relapse after treatment is discontinued.
Candida species
The preferred initial therapy for candidal meningitis is amphotericin B (0.7 mg/kg/day). Flucytosine (25 mg/kg every 6 hours) is usually added and adjusted to maintain serum levels of 40-60 µg/mL. Azoles may be used for follow-up therapy or suppressive treatment.
The risk of relapse is high, and the duration of treatment is arbitrary. Some recommend continuing treatment for a minimum of 4 weeks after the complete resolution of symptoms. The removal of prosthetic materials (eg, ventriculoperitoneal shunts) is a significant component of therapy in candidal meningitis associated with neurosurgical procedures.
Sporothrix schenckii
The lipid formulation of amphotericin B is the recommended initial treatment; after the patient responds, itraconazole (200 mg twice daily) is recommended as step-down therapy and should be given to complete a total of at least 12 months of therapy. Using itraconazole to achieve lifelong suppression may be attempted after initial therapy with amphotericin B. Fluconazole is less active againstSporothrix than itraconazole is.
Treatment of Tuberculous Meningitis
Treatment of tuberculous meningitis with a combination of first-line drugs is advocated. The selection depends on the resistance pattern in the community and the results of susceptibility testing (once available). Isoniazid and pyrazinamide attain good CSF levels (approximating blood levels). Rifampin penetrates the blood-brain barrier less efficiently but still attains adequate CSF levels. Ethambutol and streptomycin may also be part of combination therapy.
The dosages of drugs for tuberculous meningitis are similar to those used for pulmonary tuberculosis, as follows:
- Isoniazid 300 mg/day
- Rifampin 600 mg/day
- Pyrazinamide 15-30 mg/kg/day
- Ethambutol 15-25 mg/kg/day
- Streptomycin 7.5 mg/kg every 12 hours
The recommended duration of treatment is 9-12 months.
Corticosteroid therapy is indicated for patients with stage 2 or stage 3 disease (ie, those with evidence of neurologic deficits or deterioration in mental function). The rationale lies in the reduction of inflammatory effects associated with mycobacterial killing by the antimicrobial agents. The agent usually chosen is dexamethasone; the recommended dose is 60-80 mg/day, which may be tapered gradually during a span of 6 weeks.
Treatment of Syphilitic Meningitis
The treatment of choice for neurosyphilis is aqueous crystalline penicillin G (2-4 million U/day IV every 4 hours for 10-14 days), often followed with IM penicillin G benzathine (2.4 million U). An alternative is procaine penicillin G (2.4 million U/day IM) plus probenecid (500 mg orally every 6 hours for 14 days), followed by IM benzathine penicillin G (2.4 million U). These regimens are also used for neurosyphilis in patients with HIV infection. Because penicillin G is the treatment of choice, penicillin-allergic patients should undergo penicillin desensitization.
After treatment, CSF examination is repeated regularly (eg, every 6 months) to document the success of therapy. Failure of the cell count to normalize or the serologic titers to fall may warrant retreatment.
Treatment of Parasitic Meningitis
Primary amebic meningoencephalitis (PAM), caused by N fowleri, is usually fatal. The few survivors reported in the scientific literature benefited from early diagnosis and treatment with high-dose IV and intrathecal amphotericin B or miconazole and rifampin.
Treatment of helminthic eosinophilic meningitis (such as that caused by A cantonensis or G spinigerum) is largely supportive. It includes adequate analgesia, therapeutic CSF aspiration, and the use of anti-inflammatory agents, such as corticosteroids. Anthelmintic therapy may be contraindicated, because clinical deterioration and death may occur as a consequence of severe inflammatory reactions to the dying worms.
Treatment of Lyme Meningitis
Ideally, neurologic complications of Lyme disease (other than Bell palsy) are treated with parenteral antibiotics. The drug of choice is ceftriaxone (2 g/day for 14-28 days). The alternative therapy is penicillin G (20 million U/day for 14-28 days). Doxycycline (100 mg orally or IV every 12 hours for 14-28 days) or chloramphenicol (1 g every 6 hours for 14-28 days) has also been used. Treatment for only 10 days has been associated with a high rate of residual symptoms.
Prevention
Vaccination and chemoprophylaxis are 2 means of preventing meningitis.
H influenzae vaccine
Vaccination against H influenzae type B (Hib) is strongly recommended in susceptible individuals (though there is no standard recommendation for H influenzae vaccination in adults). Vaccination against S pneumoniae is also strongly encouraged for susceptible individuals, including people older than 65 years and individuals with chronic cardiopulmonary illnesses. It is not known whether the adult use of conjugate pneumococcal vaccine decreases the incidence of S pneumoniaemeningitis.
N meningitidis serogroups A, C, Y, and W-135 vaccine
Vaccinations against encapsulated bacterial organisms (eg, S pneumoniae and N meningitidis) are encouraged for people with functional or structural asplenia. Vaccinations should always be administered expeditiously to individuals who undergo splenectomy.
Vaccination with quadrivalent meningococcal polysaccharide vaccine should be offered to all high-risk populations, including those who have underlying immune deficiencies, those who travel to hyperendemic areas and epidemic areas, and those who do laboratory work that involves routine exposure to N meningitidis.College students who live in dormitories or residence halls are at modest risk; they should be informed about the risk and offered vaccination.
One vaccine protects against 4 strains of N meningitidis. As of February 2008, the CDC Advisory Committee on Immunization Practices (ACIP) no longer recommends routine immunization of children with this vaccine, but the ACIP continues to recommend routine immunization of teenagers and all children or adults at increased risk.
In 2010, the ACIP issued updated recommendations for the use of meningococcal conjugate vaccines. Two recommendations focus on the routine vaccination of adolescents and on a primary series of vaccinations of persons aged 2-55 years with certain risk factors for meningococcal infection.
Regarding the routine use of vaccines in adolescents, the 2010 CDC-ACIP guidelines specifically recommend 1 dose of meningococcal conjugate vaccine, preferably starting at 11 or 12 years. A booster dose should be given at age 16 years. If the primary dose was at age 13-15 years, the booster can be given at age 16-18 years. No booster is needed if the primary dose was given at age 16 years or later.
Regarding specific recommendations for individuals with certain risk factors for meningococcal infection, the ACIP stated that HIV-infected individuals aged 11-18 years should be given a primary series of 2 doses, 2 months apart. This should be followed by a booster dose administered at age 16 years (if the primary dose was at age 11 or 12) or at age 16-18 years (if the primary dose was at age 13-15 years). No booster is needed if the primary dose was given at age 16 years or later.
Persons aged 2-55 years who have persistent complement component deficiency or asplenia (functional or anatomic) should be given a primary series of 2 doses, 2 months apart, followed by a booster dose every 5 years. If a 1-dose primary series was given, the booster dose should be given as soon as possible, then every 5 years thereafter.
In persons aged 2-55 years with a protracted increased risk for exposure to meningitis, the 2010 ACIP guidelines recommend a 1-dose primary series. The booster dose should be given after 3 years for children aged 2-6 years and after 5 years for persons aged 7 years or older, if the person remains at increased risk.
N meningitidis serogroup B vaccine
According to the CDC, in 2012, approximately 500 cases of meningococcal disease were reported; of those, 160 resulted from serogroup B.
In October 2014, the FDA approved the first meningococcal vaccine for serogroup B (Trumenba) under the breakthrough therapy designation and accelerated approval regulatory pathways. Recent outbreaks of serogroup B meningococcal disease on a few college campuses have heightened concerns for this potentially deadly disease.
Approval was based on 3 randomized trials conducted in the United States and Europe in about 2800 adolescents. Among participants who were given 3 doses of the vaccine, 82% developed antibodies against 4 different N meningitidis serogroup B strains representative of those that cause serogroup B meningococcal disease in the United States compared with less than 1% before vaccination.
In January 2015, a second meningococcal serogroup B vaccine was approved (Bexsero).
Pneumococcal vaccine
The ACIP recommends administration of 13-valent pneumococcal polysaccharide-protein conjugate vaccine as part of routine childhood immunization.The ACIP recommends targeted use of the 23-valent pneumococcal polysaccharide vaccine (PPSV23, formerly PPV23) in children aged 2-18 years with underlying medical conditions that increase the risk of pneumococcal disease or complications. Vaccination against measles and mumps effectively eliminates aseptic meningitis syndrome caused by these pathogens. In September 2014, the CDC recommended that all adults aged 65 years or older receive both PCV13 (13-valent pneumococcal conjugate vaccine) and PPSV23 (23-valent pneumococcal polysaccharide vaccine) as part of routine vaccination.
Chemoprophylaxis
After exposure to an index case involving H influenzae, N meningitidis, or S pneumoniae, temporary nasopharyngeal carriage of the organism is typical. An association between carriage and the risk of disease has been described, especially for N meningitidis and H influenzae. This is the basis for the recommendations on chemoprophylaxis. However, such prophylaxis does not treat incubating invasive disease; accordingly close monitoring of individuals at highest risk is crucial.
To eliminate nasopharyngeal carriage of Hib and to decrease invasion of colonized susceptible individuals, rifampin (20 mg/kg/day for 4 days) is given. The index patient may need chemoprophylaxis if the administered treatment does not eliminate carriage.
Prophylaxis is suggested for contacts of persons with meningococcal meningitis (eg, household contacts, daycare center members who eat and sleep in the same dwelling, close contacts in military barracks or boarding schools, and medical personnel performing mouth-to-mouth resuscitation). Rifampin (600 mg PO every 12 hours for 2 days) can rapidly eradicate the carrier stage, and the prophylaxis persists for as long as 10 weeks after treatment.
Alternative agents for adults include ceftriaxone (250 mg IM in a single dose); this agent is also the safest choice in pregnant patients. Ceftriaxone has been shown to eradicate the carrier state for 14 days. Ciprofloxacin (500-750 mg in a single dose) is also effective.
Consultations
Consultation with an infectious diseases specialist is indicated. Consultation with a neurosurgeon is indicated in patients with any of the following:
- Severe intracranial hypertension
- Evidence of paranasal and mastoid infection that warrants surgical drainage
- Skull fractures
- Foreign body–associated infections (eg, ventriculoperitoneal shunts)
- Associated abscess formation
Long-Term Monitoring
Vigilant surveillance for the development of complications is required in patients with meningitis. Seizure precautions are indicated, especially for patients with impaired mental function. Proper isolation precautions are indicated in cases of invasive meningococcal disease.
Patients must be monitored for potential adverse effects of medications, such as hypersensitivity reactions, cytopenia, or liver dysfunction. Drug-level monitoring may be needed for some antibiotics (eg, vancomycin and the aminoglycosides).
Medication Summary
Begin empiric antibiotic coverage according to age and presence of overriding physical conditions. Empiric therapy also depends on prevalence of cephalosporin-resistant S pneumoniae (DRSP). In the United States, prevalence is considered high (>2-5%). Patients with severe penicillin (and presumed cephalosporin) allergies often require alternative therapy.
 RSS Feed
RSS Feed Twitter
Twitter


 Saturday, March 28, 2015
Saturday, March 28, 2015
 Unknown
Unknown
 Posted in
Posted in
I really appreciate DR AKHIGBE,my name is LAURIE HUGHES . I will never stop testifying DR AKHIGBE , Happiness is all i see now I never thought that I will be cured from HIV virus again. DR AKHIGBE did it for me I have been suffering from a deadly disease (HIV) for the past 2 years now, I had spent a lot of money going from one place to another, from churches to churches, hospitals have been my home every day residence. Constant checks up have been my hobby not until this faithful day, I saw a testimony on how DR AKHIGBE helped someone in curing his HIV disease in internet quickly I copied his email which is drrealakhigbe@gmail.com just to give him a test I spoke to him, he asked me to do some certain things which I did, he told me that he is going to provide the herbal cure to me, which he did, then he asked me to go for medical checkup after some days, after using the herbal cure and i did, behold I was free from the deadly disease,till now no HIV in me again he only asked me to post the testimony through the whole world, faithfully am doing it now,all the testimony of DR AKHIGBE is true please BROTHER and SISTER, MOTHER and FATHER he is great, I owe him in return. if you are having a similar problem just email him on drrealakhigbe@gmail.com or you can whats App his mobile number on +2348142454860 He can also cure these diseases like HIV and AIDS HERPES,DIABETICS,CANCER, HEPATITIS A&B,CHRONIC DISEASES, ASTHMA, HEART DISEASES, EXTERNAL INFECTION, EPILEPSY, STROKE, MULTIPLE SCLEROSIS, NAUSEA,VOMITING OR DIARRHEA,PARKINSON DISEASE,INFLUENZA,. COMMON COLD, AUTOIMMUNE DISORDER, MENINGITIS, LUPUS,ECZEMA,BACK PAIN, JOINT SCHIZOPHRENIA , PAIN.LOWER RESPIRATORY INFECTION. .ETC .please email drrealakhigbe@gmail.com or whats APP him ..+2348142454860 he is a real good and honest man.
ReplyDeletewebsite... https:drrealakhigbe.weebly.com
I started on COPD Herbal treatment from Ultimate Health Home, the treatment worked incredibly for my lungs condition. I used the herbal treatment for almost 4 months, it reversed my COPD. My severe shortness of breath, dry cough, chest tightness gradually disappeared. Reach Ultimate Health Home via their email at ultimatehealthhome@gmail.com . I can breath much better and It feels comfortable!
ReplyDelete