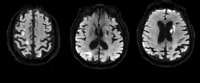
Ischemic stroke (see the image below) is characterized by the sudden loss of blood circulation to an area of the brain, resulting in a corresponding loss of neurologic function. Acute ischemic stroke is caused by thrombotic or embolic occlusion of a cerebral artery and is more common than hemorrhagic stroke.
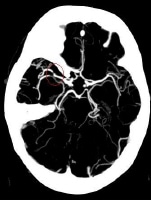 Maximum intensity projection (MIP) image from a computed tomography angiogram (CTA) demonstrates a filling defect or high-grade stenosis at the branching point of the right middle cerebral artery (MCA) trunk (red circle), suspicious for thrombus or embolus. CTA is highly accurate in detecting large- vessel stenosis and occlusions, which account for approximately one third of ischemic strokes.
Maximum intensity projection (MIP) image from a computed tomography angiogram (CTA) demonstrates a filling defect or high-grade stenosis at the branching point of the right middle cerebral artery (MCA) trunk (red circle), suspicious for thrombus or embolus. CTA is highly accurate in detecting large- vessel stenosis and occlusions, which account for approximately one third of ischemic strokes.Essential update: Study indicates intra-arterial treatment improves functional recovery in acute ischemic stroke
A randomized clinical trial from the Netherlands indicates that endovascular intervention benefits functional outcomes in patients with acute ischemic stroke resulting from a proximal intracranial arterial occlusion. In the study, by Berkhemer et al, 500 patients were randomized to receive intra-arterial treatment (within 6 hours of symptom onset) or usual treatment alone. Although 89% of the patients were treated with tissue-type plasminogen activator (t-PA) prior to randomization, only those who were found on computed tomography (CT) angiography to still have a proximal arterial occlusion were entered into the study. In most of the patients who received intra-arterial treatment, the procedure was performed with latest-generation stent retrievers.[1, 2]
The investigators found that at 90 days, the rate of functional independence (modified Rankin scale score of 0-2) in the intra-arterial treatment group was 32.6%, compared with 19.1% in the usual-treatment-only group, while after 5-7 days, the National Institutes of Health Stroke Scale (NIHSS) score was an average of 2.9 points lower in the intra-arterial treatment patients than it was in the other group. The two groups did not differ significantly with regard to the rate of morality or symptomatic intracerebral hemorrhage.
Signs and symptoms
Consider stroke in any patient presenting with acute neurologic deficit or any alteration in level of consciousness. Common stroke signs and symptoms include the following:
- Abrupt onset of hemiparesis, monoparesis, or (rarely) quadriparesis
- Hemisensory deficits
- Monocular or binocular visual loss
- Visual field deficits
- Diplopia
- Dysarthria
- Facial droop
- Ataxia
- Vertigo (rarely in isolation)
- Nystagmus
- Aphasia
- Sudden decrease in level of consciousness
Although such symptoms can occur alone, they are more likely to occur in combination. No historical feature distinguishes ischemic from hemorrhagic stroke, although nausea, vomiting, headache, and sudden change in level of consciousness are more common in hemorrhagic strokes. In younger patients, a history of recent trauma, coagulopathies, illicit drug use (especially cocaine), migraines, or use of oral contraceptives should be elicited.
With the availability of fibrinolytic therapy for acute ischemic stroke in selected patients, the physician must be able to perform a brief but accurate neurologic examination on patients with suspected stroke syndromes. The goals of the neurologic examination include the following:
- Confirming the presence of a stroke syndrome
- Distinguishing stroke from stroke mimics
- Establishing a neurologic baseline, should the patient's condition improve or deteriorate
- Establishing stroke severity, using a structured neurologic exam and score (National Institutes of Health Stroke Scale [NIHSS]) to assist in prognosis and therapeutic selection
Essential components of the neurologic examination include the following evaluations:
- Cranial nerves
- Motor function
- Sensory function
- Cerebellar function
- Gait
- Deep tendon reflexes
- Language (expressive and receptive capabilities)
- Mental status and level of consciousness
The skull and spine also should be examined, and signs of meningismus should be sought.
Diagnosis
Emergent brain imaging is essential for confirming the diagnosis of ischemic stroke. Noncontrast computed tomography (CT) scanning is the most commonly used form of neuroimaging in the acute evaluation of patients with apparent acute stroke. The following neuroimaging techniques are also used:
- CT angiography and CT perfusion scanning
- Magnetic resonance imaging (MRI)
- Carotid duplex scanning
- Digital subtraction angiography
Lumbar puncture
A lumbar puncture is required to rule out meningitis or subarachnoid hemorrhage when the CT scan is negative but the clinical suspicion remains high
Laboratory studies
Laboratory tests performed in the diagnosis and evaluation of ischemic stroke include the following:
- Complete blood count (CBC): A baseline study that may reveal a cause for the stroke (eg, polycythemia, thrombocytosis, thrombocytopenia, leukemia) or provide evidence of concurrent illness (eg, anemia)
- Basic chemistry panel: A baseline study that may reveal a stroke mimic (eg, hypoglycemia, hyponatremia) or provide evidence of concurrent illness (eg, diabetes, renal insufficiency)
- Coagulation studies: May reveal a coagulopathy and are useful when fibrinolytics or anticoagulants are to be used
- Cardiac biomarkers: Important because of the association of cerebral vascular disease and coronary artery disease
- Toxicology screening: May assist in identifying intoxicated patients with symptoms/behavior mimicking stroke syndromes
- Pregnancy testing: A urine pregnancy test should be obtained for all women of childbearing age with stroke symptoms; recombinant tissue-type plasminogen activator (rt-PA) is a pregnancy class C agent
- Arterial blood gas analysis: In selected patients with suspected hypoxemia, arterial blood gas defines the severity of hypoxemia and may be used to detect acid-base disturbances
Management
The goal for the emergent management of stroke is to complete the following within 60 minutes of patient arrival :
- Assess airway, breathing, and circulation (ABCs) and stabilize the patient as necessary
- Complete the initial evaluation and assessment, including imaging and laboratory studies
- Initiate reperfusion therapy, if appropriate
Critical treatment decisions focus on the following:
- The need for airway management
- Optimal blood pressure control
- Identifying potential reperfusion therapies (eg, intravenous fibrinolysis with rt-PA or intra-arterial approaches)
Involvement of a physician with a special interest in stroke is ideal. Stroke care units with specially trained personnel exist and improve outcomes.
Ischemic stroke therapies include the following:
- Fibrinolytic therapy
- Antiplatelet agents
- Mechanical thrombectomy
Treatment of comorbid conditions may include the following:
- Reduce fever
- Correct hypotension/significant hypertension
- Correct hypoxia
- Correct hypoglycemia
- Manage cardiac arrhythmias
- Manage myocardial ischemia
Stroke prevention
Primary stroke prevention refers to the treatment of individuals with no previous history of stroke. Measures may include use of the following:
- Platelet antiaggregants
- Statins
- Exercise
- Lifestyle interventions (eg, smoking cessation, alcohol moderation)
Secondary prevention refers to the treatment of individuals who have already had a stroke. Measures may include use of the following:
- Platelet antiaggregants
- Antihypertensives
- Statins
- Lifestyle interventions
Background
Acute ischemic stroke (AIS) is characterized by the sudden loss of blood circulation to an area of the brain, typically in a vascular territory, resulting in a corresponding loss of neurologic function. Also previously called cerebrovascular accident (CVA) or stroke syndrome, stroke is a nonspecific state of brain injury with neuronal dysfunction that has several pathophysiologic causes. Strokes can be divided into 2 types: hemorrhagic or ischemic. Acute ischemic stroke is caused by thrombotic or embolic occlusion of a cerebral artery. (See the image below.)
 Maximum intensity projection (MIP) image from a computed tomography angiogram (CTA) demonstrates a filling defect or high-grade stenosis at the branching point of the right middle cerebral artery (MCA) trunk (red circle), suspicious for thrombus or embolus. CTA is highly accurate in detecting large- vessel stenosis and occlusions, which account for approximately one third of ischemic strokes.
Maximum intensity projection (MIP) image from a computed tomography angiogram (CTA) demonstrates a filling defect or high-grade stenosis at the branching point of the right middle cerebral artery (MCA) trunk (red circle), suspicious for thrombus or embolus. CTA is highly accurate in detecting large- vessel stenosis and occlusions, which account for approximately one third of ischemic strokes.
Nearly 800,000 people suffer strokes each year in the United States; 82-92% of these strokes are ischemic. Stroke is the fourth leading cause of adult death and disability, resulting in over $72 billion in annual cost.
Ischemic and hemorrhagic stroke cannot be reliably differentiated on the basis of clinical examination findings alone. Further evaluation, especially with brain imaging tests (ie, computed tomography [CT] scanning or magnetic resonance imaging [MRI]), is required. (See Workup.)
Stroke categories
The system of categorizing stroke developed in the multicenter Trial of ORG 10172 in Acute Stroke Treatment (TOAST) divides ischemic strokes into the following 3 major subtypes :
- Large-artery
- Small-vessel, or lacunar
- Cardioembolic infarction
Large-artery infarctions often involve thrombotic in situ occlusions on atherosclerotic lesions in the carotid, vertebrobasilar, and cerebral arteries, typically proximal to major branches; however, large-artery infarctions may also be cardioembolic.
Cardiogenic emboli are a common source of recurrent stroke. They may account for up to 20% of acute strokes and have been reported to have the highest 1-month mortality.
Treatment
Recanalization strategies, including intravenous recombinant tissue-type plasminogen activator (rt-PA) and intra-arterial approaches, attempt to establish revascularization so that cells in the ischemic penumbra (a metabolically active region, peripheral to the ischemic area, where blood flow is reduced) can be rescued before irreversible injury occurs. Restoring blood flow can mitigate the effects of ischemia only if performed quickly.
The US Food and Drug Administration (FDA) has approved the use of rt-PA in patients who meet criteria set forth by the National Institute of Neurologic Disorders and Stroke (NINDS). In particular, rt-PA must be given within 3 hours of stroke onset and only after CT scanning has ruled out hemorrhagic stroke.
On the basis of recent European data, the American Heart Association and American Stroke Association recommended expanding the window of treatment from 3 hours to 4.5 hours, with more stringent exclusion criteria for the later period (see Treatment). The FDA has not yet approved rt-PA for this expanded indication, but this has become the community standard in many institutions.
Other aspects of treatment for acute ischemic stroke include the following:
Treatment of neurologic complications
- Supplemental oxygen as required
- Antiplatelet therapy
- Glycemic control
- Optimal blood pressure control
- Prevention of hyperthermia
Anatomy
The brain is the most metabolically active organ in the body. While representing only 2% of the body's mass, it requires 15-20% of the total resting cardiac output to provide the necessary glucose and oxygen for its metabolism.
Knowledge of cerebrovascular arterial anatomy and the territories supplied by the cerebral arteries is useful in determining which vessels are involved in acute stroke. Atypical patterns of brain ischemia that do not conform to specific vascular distributions may indicate a diagnosis other than ischemic stroke, such as venous infarction.
Arterial distributions
In a simplified model, the cerebral hemispheres are supplied by 3 paired major arteries, specifically, the anterior, middle, and posterior cerebral arteries.
The anterior and middle cerebral arteries carry the anterior circulation and arise from the supraclinoid internal carotid arteries. The anterior cerebral artery (ACA) supplies the medial portion of the frontal and parietal lobes and anterior portions of basal ganglia and anterior internal capsule. (See the image below.)
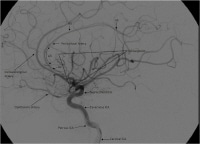 Lateral view of a cerebral angiogram illustrates the branches of the anterior cerebral artery (ACA) and Sylvian triangle. The pericallosal artery has been described to arise distal to the anterior communicating artery or distal to the origin of the callosomarginal branch of the ACA. The segmental anatomy of the ACA has been described as follows: the A1 segment extends from the internal carotid artery (ICA) bifurcation to the anterior communicating artery; A2 extends to the junction of the rostrum and genu of the corpus callosum; A3 extends into the bend of the genu of the corpus callosum; A4 and A5 extend posteriorly above the callosal body and superior portion of the splenium. The Sylvian triangle overlies the opercular branches of the middle cerebral artery (MCA), with the apex representing the Sylvian point.
Lateral view of a cerebral angiogram illustrates the branches of the anterior cerebral artery (ACA) and Sylvian triangle. The pericallosal artery has been described to arise distal to the anterior communicating artery or distal to the origin of the callosomarginal branch of the ACA. The segmental anatomy of the ACA has been described as follows: the A1 segment extends from the internal carotid artery (ICA) bifurcation to the anterior communicating artery; A2 extends to the junction of the rostrum and genu of the corpus callosum; A3 extends into the bend of the genu of the corpus callosum; A4 and A5 extend posteriorly above the callosal body and superior portion of the splenium. The Sylvian triangle overlies the opercular branches of the middle cerebral artery (MCA), with the apex representing the Sylvian point.
The middle cerebral artery (MCA) supplies the lateral portions of the frontal and parietal lobes, as well as the anterior and lateral portions of the temporal lobes, and gives rise to perforating branches to the globus pallidus, putamen, and internal capsule. The MCA is the dominant source of vascular supply to the hemispheres. (See the images below.)
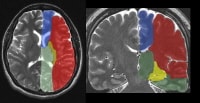 The supratentorial vascular territories of the major cerebral arteries are demonstrated superimposed on axial (left) and coronal (right) T2-weighted images through the level of the basal ganglia and thalami. The middle cerebral artery (MCA; red) supplies the lateral aspects of the hemispheres, including the lateral frontal, parietal, and anterior temporal lobes; insula; and basal ganglia. The anterior cerebral artery (ACA; blue) supplies the medial frontal and parietal lobes. The posterior cerebral artery (PCA; green) supplies the thalami and occipital and inferior temporal lobes. The anterior choroidal artery (yellow) supplies the posterior limb of the internal capsule and part of the hippocampus extending to the anterior and superior surface of the occipital horn of the lateral ventricle.
The supratentorial vascular territories of the major cerebral arteries are demonstrated superimposed on axial (left) and coronal (right) T2-weighted images through the level of the basal ganglia and thalami. The middle cerebral artery (MCA; red) supplies the lateral aspects of the hemispheres, including the lateral frontal, parietal, and anterior temporal lobes; insula; and basal ganglia. The anterior cerebral artery (ACA; blue) supplies the medial frontal and parietal lobes. The posterior cerebral artery (PCA; green) supplies the thalami and occipital and inferior temporal lobes. The anterior choroidal artery (yellow) supplies the posterior limb of the internal capsule and part of the hippocampus extending to the anterior and superior surface of the occipital horn of the lateral ventricle.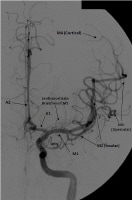 Frontal view of a cerebral angiogram with selective injection of the left internal carotid artery (ICA) illustrates the anterior circulation. The anterior cerebral artery (ACA) consists of the A1 segment proximal to the anterior communicating artery, with the A2 segment distal to it. The middle cerebral artery (MCA) can be divided into 4 segments: the M1 (horizontal segment) extends to the anterior basal portion of the insular cortex (the limen insulae) and gives off lateral lenticulostriate branches, the M2 (insular segment), M3 (opercular branches), and M4 (distal cortical branches on the lateral hemispheric convexities).
Frontal view of a cerebral angiogram with selective injection of the left internal carotid artery (ICA) illustrates the anterior circulation. The anterior cerebral artery (ACA) consists of the A1 segment proximal to the anterior communicating artery, with the A2 segment distal to it. The middle cerebral artery (MCA) can be divided into 4 segments: the M1 (horizontal segment) extends to the anterior basal portion of the insular cortex (the limen insulae) and gives off lateral lenticulostriate branches, the M2 (insular segment), M3 (opercular branches), and M4 (distal cortical branches on the lateral hemispheric convexities).
The posterior cerebral arteries arise from the basilar artery and carry the posterior circulation. The posterior cerebral artery (PCA) gives rise to perforating branches that supply the thalami and brainstem and the cortical branches to the posterior and medial temporal lobes and occipital lobes. (See Table 1, below.)
The cerebellar hemispheres are supplied as follows:
- Inferiorly by the posterior inferior cerebellar artery (PICA), arising from the vertebral artery (see the image below)
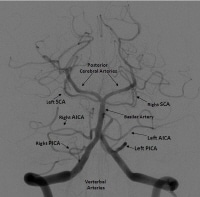 Frontal projection from a right vertebral artery angiogram illustrates the posterior circulation. The vertebral arteries join to form the basilar artery. The posterior inferior cerebellar arteries (PICAs) arise from the distal vertebral arteries. The anterior inferior cerebellar arteries (AICAs) arise from the proximal basilar artery. The superior cerebellar arteries (SICAs) arise distally from the basilar artery prior to its bifurcation into the posterior cerebral arteries (PCAs).
Frontal projection from a right vertebral artery angiogram illustrates the posterior circulation. The vertebral arteries join to form the basilar artery. The posterior inferior cerebellar arteries (PICAs) arise from the distal vertebral arteries. The anterior inferior cerebellar arteries (AICAs) arise from the proximal basilar artery. The superior cerebellar arteries (SICAs) arise distally from the basilar artery prior to its bifurcation into the posterior cerebral arteries (PCAs). - Superiorly by the superior cerebellar artery
- Anterolaterally by the anterior inferior cerebellar artery (AICA), from the basilar artery
Table 1. Vascular Supply to the Brain
| VASCULAR TERRITORY | Structures Supplied |
| Anterior Circulation (Carotid) | |
| Anterior Cerebral Artery | Cortical branches: medial frontal and parietal lobe Medial lenticulostriate branches: caudate head, globus pallidus, anterior limb of internal capsule |
| Middle Cerebral Artery | Cortical branches: lateral frontal and parietal lobes lateral and anterior temporal lobe Lateral lenticulostriate branches: globus pallidus and putamen, internal capsule |
| Anterior Choroidal Artery | Optic tracts, medial temporal lobe, ventrolateral thalamus, corona radiata, posterior limb of the internal capsule |
| Posterior Circulation (Vertebrobasilar) | |
| Posterior Cerebral Artery | Cortical branches: occipital lobes, medial and posterior temporal and parietal lobes Perforating branches: brainstem, posterior thalamus and midbrain |
| Posterior Inferior Cerebellar Artery | Inferior vermis; posterior and inferior cerebellar hemispheres |
| Anterior Inferior Cerebellar Artery | Anterolateral cerebellum |
| Superior Cerebellar Artery | Superior vermis; superior cerebellum |
Pathophysiology
Acute ischemic strokes result from vascular occlusion secondary to thromboembolic disease (see Etiology). Ischemia causes cell hypoxia and depletion of cellular adenosine triphosphate (ATP). Without ATP, there is no longer the energy to maintain ionic gradients across the cell membrane and cell depolarization. Influx of sodium and calcium ions and passive inflow of water into the cell lead to cytotoxic edema.
Ischemic core and penumbra
An acute vascular occlusion produces heterogeneous regions of ischemia in the affected vascular territory. Local blood flow is limited to any residual flow in the major arterial source plus the collateral supply, if any.
Affected regions with cerebral blood flow of lower than 10 mL/100 g of tissue/min are referred to collectively as the core. These cells are presumed to die within minutes of stroke onset.
Zones of decreased or marginal perfusion (cerebral blood flow < 25 mL/100g of tissue/min) are collectively called the ischemic penumbra. Tissue in the penumbra can remain viable for several hours because of marginal tissue perfusion.
Ischemic cascade
On the cellular level, the ischemic neuron becomes depolarized as ATP is depleted and membrane ion-transport systems fail. Disruption of cellular metabolism also impairs normal sodium-potassium plasma membrane pumps, producing an intracellular increase in sodium, which in turns increases intracellular water content. This cellular swelling is referred to as cytotoxic edema and occurs very early in cerebral ischemia.
Cerebral ischemia impairs the normal sodium-calcium exchange protein also found on cell plasma membranes. The resulting influx of calcium leads to the release of a number of neurotransmitters, including large quantities of glutamate, which in turn activates N -methyl-D-aspartate (NMDA) and other excitatory receptors on other neurons.
These neurons then become depolarized, causing further calcium influx, further glutamate release, and local amplification of the initial ischemic insult. This massive calcium influx also activates various degradative enzymes, leading to the destruction of the cell membrane and other essential neuronal structures. Free radicals, arachidonic acid, and nitric oxide are generated by this process, which leads to further neuronal damage.
Ischemia also directly results in dysfunction of the cerebral vasculature, with breakdown of the blood-brain barrier occurring within 4-6 hours after infarction. Following the barrier’s breakdown, proteins and water flood into the extracellular space, leading to vasogenic edema. This produces greater levels of brain swelling and mass effect that peak at 3-5 days and resolve over the next several weeks with resorption of water and proteins.
Within hours to days after a stroke, specific genes are activated, leading to the formation of cytokines and other factors that, in turn, cause further inflammation and microcirculatory compromise. Ultimately, the ischemic penumbra is consumed by these progressive insults, coalescing with the infarcted core, often within hours of the onset of the stroke.
Infarction results in the death of astrocytes, as well as the supporting oligodendroglial and microglial cells. The infarcted tissue eventually undergoes liquefaction necrosis and is removed by macrophages, with the development of parenchymal volume loss. A well-circumscribed region of cerebrospinal fluid–like low density, resulting from encephalomalacia and cystic change, is eventually seen. The evolution of these chronic changes may be seen in the weeks to months following the infarction. (See the images below.)
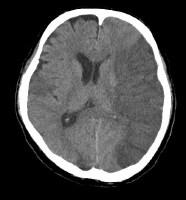 Vascular distributions: Middle cerebral artery (MCA) infarction. Noncontrast computed tomography (CT) scanning demonstrates a large acute infarction in the MCA territory involving the lateral surfaces of the left frontal, parietal, and temporal lobes, as well as the left insular and subinsular regions, with mass effect and rightward midline shift. There is sparing of the caudate head and at least part of the lentiform nucleus and internal capsule, which receive blood supply from the lateral lenticulostriate branches of the M1 segment of the MCA. Note the lack of involvement of the medial frontal lobe (anterior cerebral artery [ACA] territory), thalami, and paramedian occipital lobe (posterior cerebral artery [PCA] territory).
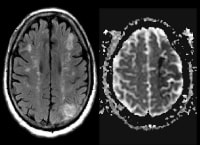
Vascular distributions: Middle cerebral artery (MCA) infarction. Noncontrast computed tomography (CT) scanning demonstrates a large acute infarction in the MCA territory involving the lateral surfaces of the left frontal, parietal, and temporal lobes, as well as the left insular and subinsular regions, with mass effect and rightward midline shift. There is sparing of the caudate head and at least part of the lentiform nucleus and internal capsule, which receive blood supply from the lateral lenticulostriate branches of the M1 segment of the MCA. Note the lack of involvement of the medial frontal lobe (anterior cerebral artery [ACA] territory), thalami, and paramedian occipital lobe (posterior cerebral artery [PCA] territory).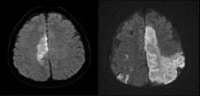 Vascular distributions: Anterior cerebral artery (ACA) infarction. Diffusion-weighted image on the left demonstrates high signal in the paramedian frontal and high parietal regions. The opposite diffusion-weighted image in a different patient demonstrates restricted diffusion in a larger ACA infarction involving the left paramedian frontal and posterior parietal regions. There is also infarction of the lateral temporoparietal regions bilaterally (both middle cerebral artery [MCA] distributions), greater on the left indicating multivessel involvement and suggesting emboli.
Vascular distributions: Anterior cerebral artery (ACA) infarction. Diffusion-weighted image on the left demonstrates high signal in the paramedian frontal and high parietal regions. The opposite diffusion-weighted image in a different patient demonstrates restricted diffusion in a larger ACA infarction involving the left paramedian frontal and posterior parietal regions. There is also infarction of the lateral temporoparietal regions bilaterally (both middle cerebral artery [MCA] distributions), greater on the left indicating multivessel involvement and suggesting emboli.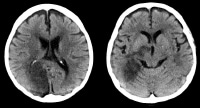 Vascular distributions: Posterior cerebral artery (PCA) infarction. The noncontrast computed tomography (CT) images demonstrate PCA distribution infarction involving the right occipital and inferomedial temporal lobes. The image on the right demonstrates additional involvement of the thalamus, also part of the PCA territory.
Vascular distributions: Posterior cerebral artery (PCA) infarction. The noncontrast computed tomography (CT) images demonstrate PCA distribution infarction involving the right occipital and inferomedial temporal lobes. The image on the right demonstrates additional involvement of the thalamus, also part of the PCA territory.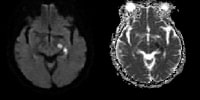 Vascular distributions: Anterior choroidal artery infarction. The diffusion-weighted image (left) demonstrates high signal with associated signal dropout on the apparent diffusion coefficient (ADC) map involving the posterior limb of the internal capsule. This is the typical distribution of the anterior choroidal artery, the last branch of the internal carotid artery (ICA) before bifurcating into the anterior and middle cerebral arteries. The anterior choroidal artery may also arise from the middle cerebral artery (MCA).
Vascular distributions: Anterior choroidal artery infarction. The diffusion-weighted image (left) demonstrates high signal with associated signal dropout on the apparent diffusion coefficient (ADC) map involving the posterior limb of the internal capsule. This is the typical distribution of the anterior choroidal artery, the last branch of the internal carotid artery (ICA) before bifurcating into the anterior and middle cerebral arteries. The anterior choroidal artery may also arise from the middle cerebral artery (MCA).Hemorrhagic transformation of ischemic stroke
Hemorrhagic transformation represents the conversion of an ischemic infarction into an area of hemorrhage. This is estimated to occur in 5% of uncomplicated ischemic strokes, in the absence of fibrinolytic treatment. Hemorrhagic transformation is not always associated with neurologic decline, with the conversion ranging from the development of small petechial hemorrhages to the formation of hematomas that produce neurologic decline and may necessitate surgical evacuation or decompressive hemicraniectomy.
Proposed mechanisms for hemorrhagic transformation include reperfusion of ischemically injured tissue, either from recanalization of an occluded vessel or from collateral blood supply to the ischemic territory or disruption of the blood-brain barrier. With disruption of the blood-brain barrier, red blood cells extravasate from the weakened capillary bed, producing petechial hemorrhage or more frank intraparenchymal hematoma.
Hemorrhagic transformation of an ischemic infarct occurs within 2-14 days postictus, usually within the first week. It is more commonly seen following cardioembolic strokes and is more likely to occur with larger infarct volumes. Hemorrhagic transformation is also more likely following administration of rt-PA in patients whose noncontrast CT (NCCT) scans demonstrate areas of hypodensity.
Poststroke cerebral edema and seizures
Although clinically significant cerebral edema can occur after anterior circulation ischemic stroke, it is thought to be somewhat rare (10-20%). Edema and herniation are the most common causes of early death in patients with hemispheric stroke.
Seizures occur in 2-23% of patients within the first days after ischemic stroke. A fraction of patients who have experienced stroke develop chronic seizure disorders.
Etiology
Ischemic strokes result from events that limit or stop blood flow, such as extracranial or intracranial thrombotic embolism, thrombosis in situ, or relative hypoperfusion. As blood flow decreases, neurons cease functioning. Although a range of thresholds has been described, irreversible neuronal ischemia and injury is generally thought to begin at blood flow rates of less than 18 mL/100 g of tissue/min, with cell death occurring rapidly at rates below 10 mL/100 g of tissue/min
Risk factors
Risk factors for ischemic stroke include modifiable and nonmodifiable conditions. Identification of risk factors in each patient can uncover clues to the cause of the stroke and the most appropriate treatment and secondary prevention plan.
Nonmodifiable risk factors include the following (although there are likely many others):
- Age
- Race
- Sex
- Ethnicity
- History of migraine headaches
- Fibromuscular dysplasia
- Heredity: Family history of stroke or transient ischemic attacks (TIAs)
In a prospective study of 27,860 women aged 45 years or older who were participating in the Women's Health Study, Kurth et al found that migraine with aura was a strong risk factor for any type of stroke. The adjusted incidence of this risk factor per 1000 women per year was similar to those of other known risk factors, including systolic blood pressure 180 mm Hg or higher, body mass index 35 kg/m2 or greater, history of diabetes, family history of myocardial infarction, and smoking.
For migraine with aura, the total incidence of stroke in the study was 4.3 per 1000 women per year, the incidence of ischemic stroke was 3.4 per 1000 per year, and the incidence of hemorrhagic stroke was 0.8 per 1000 per year.
Modifiable risk factors include the following :
- Hypertension (the most important)
- Diabetes mellitus
- Cardiac disease: Atrial fibrillation, valvular disease, heart failure, mitral stenosis, structural anomalies allowing right-to-left shunting (eg, patent foramen ovale), and atrial and ventricular enlargement
- Hypercholesterolemia
- TIAs
- Carotid stenosis
- Hyperhomocystinemia
- Lifestyle issues: Excessive alcohol intake, tobacco use, illicit drug use, physical inactivity
- Obesity
- Oral contraceptive use/postmenopausal hormone use
- Sickle cell disease
In 2014, the American Heart Association and the American Stroke Association issued guidelines for the reduction of stroke risk specifically in women. These gender-specific recommendations include the following :
- A stroke risk score should be developed specifically for women
- Women with a history of high blood pressure before pregnancy should be considered for low-dose aspirin and/or calcium supplement treatment to reduce the risk of preeclampsia
- Blood pressure medication may be considered for pregnant women with moderately high blood pressure (150-159 mmHg/100-109 mmHg), and pregnant women with severe high blood pressure (160/110 mmHg or above) should be treated
- Women should be screened for high blood pressure before they start using birth control pills because of an increased risk of stroke
- Women with migraine headaches with aura should be encouraged to quit smoking to reduce the risk of stroke
- Women over age 75 should be screened for atrial fibrillation
Genetic and inflammatory mechanisms
Evidence continues to accumulate that inflammation and genetic factors have important roles in the development of atherosclerosis and, specifically, in stroke. According to the current paradigm, atherosclerosis is not a bland cholesterol storage disease, as previously thought, but a dynamic, chronic, inflammatory condition caused by a response to endothelial injury.
Traditional risk factors, such as oxidized low-density lipoprotein (LDL) cholesterol and smoking, contribute to this injury. It has been suggested, however, that infections may also contribute to endothelial injury and atherosclerosis.
Host genetic factors, moreover, may modify the response to these environmental challenges, although inherited risk for stroke is likely multigenic. Even so, specific single-gene disorders with stroke as a component of the phenotype demonstrate the potency of genetics in determining stroke risk.
A number of genes are known to increase susceptibility to ischemic stroke. Mutations to the F2 and F5 genes are relatively common in the general population and increase the risk of thrombosis. Mutations in the following genes also are known to increase the risk of stroke:
- NOS3: A nitric oxide synthetase gene; involved in vascular relaxation
- ALOX5AP: Involved in the metabolism of arachidonic acid
- PRKCH: Involved in major signal transduction systems
Hyperhomocysteinemia and homocystinuria
Hyperhomocysteinemia is implicated in the pathogenesis of ischemic stroke. The most common concern is mutations in the 5,10-methylenetetrahydrofolate reductase (MTHFR) gene. In many populations, the mutant allele frequency reaches polymorphic proportions, and the risk factor for cerebrovascular disease is related to the serum level of homocysteine. Furthermore, in persons who are compound heterozygotes for MTHFR mutation, if elevated homocysteine is found it can be lowered with oral folic acid therapy.
In addition, hyperhomocysteinemia can be seen in cystathione beta synthetase (CBS) deficiency, which is generally referred to as homocystinuria. This disorder is inherited in an autosomal recessive manner. Symptoms usually manifest early in life. Patients have a marfanoid habitus, ectopia lentis, and myopia and generally have intellectual disability.
Thromboembolic events are the most common cause of death for patients with homocystinuria and may be of any type, including myocardial infarction. The risk of having a vascular event in homocystinuria is 50% by age 30. It was previously suggested that persons who are heterozygous for mutations in the CBS gene may have an increased risk of cerebrovascular disease as well, but several more recent studies on this subject failed to replicate this finding.
Amyloid angiopathies
Amyloid angiopathies are also known to increase risk for stroke and dementia. Mutations in the CST3 gene are causative and are inherited in an autosomal dominant manner. Sufferers will have diffuse deposition of amyloid, including in the brain. The onset of symptoms is typically in the third or fourth decade of life, with death occurring before age 60 years. These angiopathies appear to be most common in the Icelandic population.
CADASIL
Cerebral arteriopathy, autosomal dominant, with subcortical infarcts and leukoencephalopathy (CADASIL), is caused by mutations in the NOTCH3 gene. It affects the small arteries of the brain. Strokelike episodes typically occur at a mean age of 46 years, with an age range of 19-67 years. White-matter changes in the brain are typically evident by young adulthood and progress over time.
Migraine headaches occur in 30-40% of people with CADASIL. Approximately 60% of symptomatic individuals have cognitive deficits, which can start as early as age 35 years, and many develop multi-infarct dementia.
Other mutations
Genome-wide association studies have revealed additional loci that are commonly associated with ischemic stroke. Early onset ischemic stroke has been found to be associated with 2 single-nucleotide polymorphisms on 2q23.3.
Large-vessel stroke has been associated with variations in HDAC9, PITX2, andZFHX3. HDAC9 is located on7p21.1, while PITX2 and ZFHX3 are located on 9p21. It is of note that the 9p21 locus has also been associated with cardiovascular disease.
A polymorphism at 2q36.3 was found in which adenosine substitution conferred a lower risk of ischemic stroke in an additive fashion. An additional study suggested an association between ischemic stroke and a locus on 12p13.
In addition, complete information on the following metabolic diseases and stroke can be found in the following main articles:
- Methylmalonic Acidemia
- Homocystinuria/Homocysteinemia
- Fabry Disease
- MELAS Syndrome
- Hyperglycemia and Hypoglycemia in Stroke
Large-artery occlusion
Large-artery occlusion typically results from embolization of atherosclerotic debris originating from the common or internal carotid arteries or from a cardiac source. A smaller number of large-artery occlusions may arise from plaque ulceration and in situ thrombosis. Large-vessel ischemic strokes more commonly affect the MCA territory, with the ACA territory affected to a lesser degree. (See the images below.)
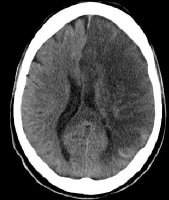 Noncontrast computed tomography (CT) scan in a 52-year-old man with a history of worsening right-sided weakness and aphasia demonstrates diffuse hypodensity and sulcal effacement with mass effect involving the left anterior and middle cerebral artery territories consistent with acute infarction. There are scattered curvilinear areas of hyperdensity noted suggestive of developing petechial hemorrhage in this large area of infarction.
Noncontrast computed tomography (CT) scan in a 52-year-old man with a history of worsening right-sided weakness and aphasia demonstrates diffuse hypodensity and sulcal effacement with mass effect involving the left anterior and middle cerebral artery territories consistent with acute infarction. There are scattered curvilinear areas of hyperdensity noted suggestive of developing petechial hemorrhage in this large area of infarction.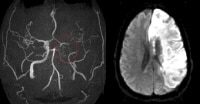 Magnetic resonance angiogram (MRA) in a 52-year-old man demonstrates occlusion of the left precavernous supraclinoid internal carotid artery (ICA, red circle), occlusion or high-grade stenosis of the distal middle cerebral artery (MCA) trunk and attenuation of multiple M2 branches. The diffusion-weighted image (right) demonstrates high signal confirmed to be true restricted diffusion on the apparent diffusion coefficient (ADC) map consistent with acute infarction.
Magnetic resonance angiogram (MRA) in a 52-year-old man demonstrates occlusion of the left precavernous supraclinoid internal carotid artery (ICA, red circle), occlusion or high-grade stenosis of the distal middle cerebral artery (MCA) trunk and attenuation of multiple M2 branches. The diffusion-weighted image (right) demonstrates high signal confirmed to be true restricted diffusion on the apparent diffusion coefficient (ADC) map consistent with acute infarction. Maximum intensity projection (MIP) image from a computed tomography angiogram (CTA) demonstrates a filling defect or high-grade stenosis at the branching point of the right middle cerebral artery (MCA) trunk (red circle), suspicious for thrombus or embolus. CTA is highly accurate in detecting large- vessel stenosis and occlusions, which account for approximately one third of ischemic strokes.
Maximum intensity projection (MIP) image from a computed tomography angiogram (CTA) demonstrates a filling defect or high-grade stenosis at the branching point of the right middle cerebral artery (MCA) trunk (red circle), suspicious for thrombus or embolus. CTA is highly accurate in detecting large- vessel stenosis and occlusions, which account for approximately one third of ischemic strokes.Lacunar strokes
Lacunar strokes represent 13-20% of all ischemic strokes. They result from occlusion of the penetrating branches of the MCA, the lenticulostriate arteries, or the penetrating branches of the circle of Willis, vertebral artery, or basilar artery. The great majority of lacunar strokes are related to hypertension. (See the image below.)
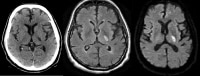 Axial noncontrast computed tomography (CT) scan demonstrates a focal area of hypodensity in the left posterior limb of the internal capsule in a 60-year-old man with acute onset of right-sided weakness. The lesion demonstrates high signal on the fluid-attenuated inversion recovery (FLAIR) sequence (middle image) and diffusion-weighted magnetic resonance imaging (MRI) scan (right image), with low signal on the apparent diffusion coefficient (ADC) maps indicating an acute lacunar infarction. Lacunar infarcts are typically no more than 1.5 cm in size and can occur in the deep gray matter structures, corona radiata, brainstem, and cerebellum.
Axial noncontrast computed tomography (CT) scan demonstrates a focal area of hypodensity in the left posterior limb of the internal capsule in a 60-year-old man with acute onset of right-sided weakness. The lesion demonstrates high signal on the fluid-attenuated inversion recovery (FLAIR) sequence (middle image) and diffusion-weighted magnetic resonance imaging (MRI) scan (right image), with low signal on the apparent diffusion coefficient (ADC) maps indicating an acute lacunar infarction. Lacunar infarcts are typically no more than 1.5 cm in size and can occur in the deep gray matter structures, corona radiata, brainstem, and cerebellum.
Causes of lacunar infarcts include the following:
- Microatheroma
- Lipohyalinosis
- Fibrinoid necrosis secondary to hypertension or vasculitis
- Hyaline arteriosclerosis
- Amyloid angiopathy
- Microemboli
Embolic strokes
Cardiogenic emboli may account for up to 20% of acute strokes. Emboli may arise from the heart, the extracranial arteries, including the aortic arch or, rarely, the right-sided circulation (paradoxical emboli) with subsequent passage through a patent foramen ovale. Sources of cardiogenic emboli include the following:
- Valvular thrombi (eg, in mitral stenosis or endocarditis or from use of a prosthetic valve)
- Mural thrombi (eg, in myocardial infarction, atrial fibrillation, dilated cardiomyopathy, or severe congestive heart failure)
- Atrial myxoma
Acute myocardial infarction is associated with a 2-3% incidence of embolic strokes, of which 85% occur in the first month after the infarction. Embolic strokes tend to have a sudden onset, and neuroimaging may demonstrate previous infarcts in several vascular territories or may show calcific emboli.
Cardioembolic strokes may be isolated, multiple and in a single hemisphere, or scattered and bilateral; the latter 2 types indicate multiple vascular distributions and are more specific for cardioembolism. Multiple and bilateral infarcts can be the result of embolic showers or recurrent emboli. Other possibilities for single and bilateral hemispheric infarctions include emboli originating from the aortic arch and diffuse thrombotic or inflammatory processes that can lead to multiple small-vessel occlusions.
 Cardioembolic stroke: Axial diffusion-weighted images demonstrate scattered foci of high signal in the subcortical and deep white matter bilaterally in a patient with a known cardiac source for embolization. An area of low signal in the left gangliocapsular region may be secondary to prior hemorrhage or subacute to chronic lacunar infarct. Recurrent strokes are most commonly secondary to cardioembolic phenomenon.
Cardioembolic stroke: Axial diffusion-weighted images demonstrate scattered foci of high signal in the subcortical and deep white matter bilaterally in a patient with a known cardiac source for embolization. An area of low signal in the left gangliocapsular region may be secondary to prior hemorrhage or subacute to chronic lacunar infarct. Recurrent strokes are most commonly secondary to cardioembolic phenomenon.Thrombotic strokes
Thrombogenic factors may include injury to and loss of endothelial cells; this loss exposes the subendothelium and results in platelet activation by the subendothelium, activation of the clotting cascade, inhibition of fibrinolysis, and blood stasis. Thrombotic strokes are generally thought to originate on ruptured atherosclerotic plaques. Arterial stenosis can cause turbulent blood flow, which can promote thrombus formation; atherosclerosis (ie, ulcerated plaques); and platelet adherence. All cause the formation of blood clots that either embolize or occlude the artery.
Intracranial atherosclerosis may be the cause of thrombotic stroke in patients with widespread atherosclerosis. In other patients, especially younger patients, other causes should be considered, including the following :
- Hypercoagulable states (eg, antiphospholipid antibodies, protein C deficiency, protein S deficiency, pregnancy)
- Sickle cell disease
- Fibromuscular dysplasia
- Arterial dissections
- Vasoconstriction associated with substance abuse (eg, cocaine, amphetamines)
Watershed infarcts
Vascular watershed, or border-zone, infarctions occur at the most distal areas between arterial territories. They are believed to be secondary to embolic phenomenon or to severe hypoperfusion, as occurs, for example, in carotid occlusion or prolonged hypotension. (See the image below.)
 Magnetic resonance imaging (MRI) scan was obtained in a 62-year-old man with hypertension and diabetes and a history of transient episodes of right-sided weakness and aphasia. The fluid-attenuated inversion recovery (FLAIR) image (left) demonstrates patchy areas of high signal arranged in a linear fashion in the deep white matter, bilaterally. This configuration is typical for deep border-zone, or watershed, infarction, in this case the anterior and posterior middle cerebral artery (MCA) watershed areas. The left-sided infarcts have corresponding low signal on the apparent diffusion coefficient (ADC) map (right), signifying acuity. An old left posterior parietal infarct is noted as well.
Magnetic resonance imaging (MRI) scan was obtained in a 62-year-old man with hypertension and diabetes and a history of transient episodes of right-sided weakness and aphasia. The fluid-attenuated inversion recovery (FLAIR) image (left) demonstrates patchy areas of high signal arranged in a linear fashion in the deep white matter, bilaterally. This configuration is typical for deep border-zone, or watershed, infarction, in this case the anterior and posterior middle cerebral artery (MCA) watershed areas. The left-sided infarcts have corresponding low signal on the apparent diffusion coefficient (ADC) map (right), signifying acuity. An old left posterior parietal infarct is noted as well.Flow disturbances
Stroke symptoms can result from inadequate cerebral blood flow because of decreased blood pressure (and specifically, decreased cerebral perfusion pressure) or as a result of hematologic hyperviscosity from sickle cell disease or other hematologic illnesses, such as multiple myeloma and polycythemia vera. In these instances, cerebral injury may occur in the presence of damage to other organ systems.
Epidemiology
Stroke is the leading cause of disability and the fourth leading cause of death in the United States. Each year, approximately 795,000 people in the United States experience new (610,000 people) or recurrent (185,000 people) stroke. Epidemiologic studies indicate that 82-92% of strokes in the United States are ischemic.
According to the World Health Organization (WHO), 15 million people suffer stroke worldwide each year. Of these, 5 million die, and another 5 million are left permanently disabled.
Race-, sex-, and age-related demographics
In the United States, blacks have an age-adjusted risk of death from stroke that is 1.49 times that of whites. Hispanics have a lower overall incidence of stroke than whites and blacks but more frequent lacunar strokes and stroke at an earlier age.
Men are at higher risk for stroke than women; white men have a stroke incidence of 62.8 per 100,000, with death being the final outcome in 26.3% of cases, while women have a stroke incidence of 59 per 100,000 and a death rate of 39.2%.
Although stroke often is considered a disease of elderly persons, one third of strokes occur in persons younger than 65 years. Risk of stroke increases with age, especially in patients older than 64 years, in whom 75% of all strokes occur.
Prognosis
In the Framingham and Rochester stroke studies, the overall mortality rate at 30 days after stroke was 28%, the mortality rate at 30 days after ischemic stroke was 19%, and the 1-year survival rate for patients with ischemic stroke was 77%. However, the prognosis after acute ischemic stroke varies greatly in individual patients, depending on the stroke severity and on the patient’s premorbid condition, age, and poststroke complications.
A study utilizing the large national Get With The Guidelines - Stroke registry found that the baseline National Institutes of Health Stroke Scale (NIHSS) score was the strongest predictor of early mortality risk, even more so than currently used mortality prediction models incorporating multiple clinical data. Cardiogenic emboli are associated with the highest 1-month mortality in patients with acute stroke.
The presence of computed tomography (CT) scan evidence of infarction early in presentation has been associated with poor outcome and with an increased propensity for hemorrhagic transformation after fibrinolytic therapy (see Pathophysiology). Hemorrhagic transformation is estimated to occur in 5% of uncomplicated ischemic strokes in the absence of fibrinolytic therapy, although it is not always associated with neurologic decline. Indeed, hemorrhagic transformation ranges from the development of small petechial hemorrhages to the formation of hematomas requiring evacuation.
Acute ischemic stroke has been associated with acute cardiac dysfunction and arrhythmia, which then correlate with worse functional outcome and morbidity at 3 months. Data suggest that severe hyperglycemia is independently associated with poor outcome and reduced reperfusion in fibrinolysis, as well as extension of the infarcted territory.
In stroke survivors from the Framingham Heart Study, 31% needed help caring for themselves, 20% needed help when walking, and 71% had impaired vocational capacity in long-term follow-up.
Patient Education
Public education must involve all age groups. Incorporating stroke into basic life support (BLS) and cardiopulmonary resuscitation (CPR) curricula is just one way to reach a younger audience. Avenues to reach an audience with a higher stroke risk could include local churches, employers, and senior organizations to promote stroke awareness.
The American Stroke Association (ASA) advises the public to be aware of the symptoms of stroke that are easily recognized, including the sudden onset of any of the following, and to call 911 immediately:
Numbness or weakness of face, arm, or leg, especially on 1 side of the body
- Confusion
- Difficulty in speaking or understanding
- Deterioration of vision in 1 or both eyes
- Difficulty in walking, dizziness, and loss of balance or coordination
- Severe headache with no known cause
History
A focused medical history for patients with ischemic stroke aims to identify risk factors for atherosclerotic and cardiac disease, including the following (see Etiology):
- Hypertension
- Diabetes mellitus
- Tobacco use
- High cholesterol
- History of coronary artery disease, coronary artery bypass, or atrial fibrillation
In younger patients, elicit a history of the following:
- Recent trauma
- Coagulopathies
- Illicit drug use (especially cocaine)
- Migraines
- Oral contraceptive use
Stroke should be considered in any patient presenting with an acute neurologic deficit (focal or global) or altered level of consciousness. No historical feature distinguishes ischemic from hemorrhagic stroke, although nausea, vomiting, headache, and a sudden change in the patient’s level of consciousness are more common in hemorrhagic strokes.
Consider stroke in any patient presenting with acute neurologic deficit or any alteration in level of consciousness. Common signs and symptoms of stroke include the abrupt onset of any of the following:
- Hemiparesis, monoparesis, or (rarely) quadriparesis
- Hemisensory deficits
- Monocular or binocular visual loss
- Visual field deficits
- Diplopia
- Dysarthria
- Facial droop
- Ataxia
- Vertigo (rarely in isolation)
- Aphasia
- Sudden decrease in the level of consciousness
Although such symptoms can occur alone, they are more likely to occur in combination.
Establishing the time at which the patient was last without stroke symptoms, or last known to be normal, is especially critical when fibrinolytic therapy is an option. Unfortunately, the median time from symptom onset to emergency department (ED) presentation ranges from 4-24 hours in the United States.
Multiple factors contribute to delays in seeking care for symptoms of stroke. Many strokes occur while patients are sleeping and are not discovered until the patient wakes (this phenomenon is also known as "wake-up" stroke). Stroke can leave some patients too incapacitated to call for help. Occasionally, a stroke goes unrecognized by patients or their caregivers.
If the patient awakens with symptoms, then the time of onset is defined as the time at which the patient was last seen to be without symptoms. Input from family members, coworkers, and bystanders may be required to help establish the exact time of onset, especially in right hemispheric strokes accompanied by neglect or left hemispheric strokes with aphasia.
Physical Examination
The goals of the physical examination are as follows:
- Detect extracranial causes of stroke symptoms
- Distinguish stroke from stroke mimics
- Determine and document for future comparison the degree of deficit
- Localize the lesion
- Identify comorbidities
- Identify conditions that may influence treatment decisions (eg, trauma, active bleeding, active infection)
The physical examination always includes a careful head and neck examination for signs of trauma, infection, and meningeal irritation. A careful search for the cardiovascular causes of stroke requires examination of the following:
- Ocular fundi (retinopathy, emboli, hemorrhage)
- Heart (irregular rhythm, murmur, gallop)
- Peripheral vasculature (palpation of carotid, radial, and femoral pulses; auscultation for carotid bruit)
The physical examination must encompass all of the major organ systems, starting with airway, breathing, and circulation (ABCs) and the vital signs. Patients with a decreased level of consciousness should be assessed to ensure that they are able to protect their airway. Patients with stroke, especially hemorrhagic stroke, can suffer quick clinical deterioration; therefore, constant reassessment is critical.
Ischemic strokes, unless large or involving the brainstem, do not tend to cause immediate problems with airway patency, breathing, or circulation compromise. On the other hand, patients with intracerebral or subarachnoid hemorrhage frequently require intervention for airway protection and ventilation.
Vital signs, while nonspecific, can point to impending clinical deterioration and may assist in narrowing the differential diagnosis. Many patients with stroke are hypertensive at baseline, and their blood pressure may become more elevated after stroke. While hypertension at presentation is common, blood pressure decreases spontaneously over time in most patients.
Head and neck, cardiac, and extremities examination
A careful examination of the head and neck is essential. Contusions, lacerations, and deformities may suggest trauma as the etiology for the patient's symptoms. Auscultation of the neck may elicit a bruit, suggesting carotid disease as the cause of the stroke.
Cardiac arrhythmias, such as atrial fibrillation, are found commonly in patients with stroke. Similarly, strokes may occur concurrently with other acute cardiac conditions, such as acute myocardial infarction and acute heart failure; thus, auscultation for murmurs and gallops is recommended.
Carotid or vertebrobasilar dissections and, less commonly, thoracic aortic dissections may cause ischemic stroke. Unequal pulses or blood pressures in the extremities may reflect the presence of aortic dissections.
Neurologic examination
With the availability of fibrinolytic therapy for acute ischemic stroke in selected patients, the physician must be able to perform a brief but accurate neurologic examination on patients with suspected stroke syndromes. The goals of the neurologic examination include the following:
- Confirming the presence of a stroke syndrome (to be defined further with cranial CT scanning)
- Distinguishing stroke from stroke mimics
- Establishing a neurologic baseline should the patient's condition improve or deteriorate
- Establishing stroke severity to assist in prognosis and therapeutic selection
Essential components of the neurologic examination include the following evaluations:
- Cranial nerves
- Motor function
- Sensory function
- Cerebellar function
- Gait
- Deep tendon reflexes
- Language (expressive and receptive capabilities)
- Mental status and level of consciousness
The skull and spine also should be examined, and signs of meningismus should be sought.
National Institutes of Health Stroke Scale
A useful tool in quantifying neurologic impairment is the National Institutes of Health Stroke Scale (NIHSS) (see Table 2, below). The NIHSS enables the healthcare provider to rapidly determine the severity and possible location of the stroke. NIHSS scores are strongly associated with outcome and can help to identify those patients who are likely to benefit from fibrinolytic therapy and those who are at higher risk of developing hemorrhagic complications of fibrinolytic use.
The NIHSS is easily performed; it focuses on the following 6 major areas of the neurologic examination:
- level of consciousness
- Visual function
- Motor function
- Sensation and neglect
- Cerebellar function
- Language
The NIHSS is a 42-point scale. Patients with minor strokes usually have a score of less than 5. An NIHSS score of greater than 10 correlates with an 80% likelihood of proximal vessel occlusions (as identified on CT or standard angiograms). However, discretion must be used in assessing the magnitude of the clinical deficit and resulting disability; for instance, if a patient's only deficit is mutism, the NIHSS score will be 3. Additionally, the scale does not measure some deficits associated with posterior circulation strokes (ie, vertigo, ataxia).
Table 2. National Institutes of Health Stroke Scale
| Category | Description | Score | |
| 1a | level of consciousness (LOC) | Alert Drowsy Stuporous Coma | 0 1 2 3 |
| 1b | LOC questions (month, age) | Answers both correctly Answers 1 correctly Incorrect on both | 0 1 2 |
| 1c | LOC commands (open and close eyes, grip and release nonparetic hand) | Obeys both correctly Obeys 1 correctly Incorrect on both | 0 1 2 |
| 2 | Best gaze (follow finger) | Normal Partial gaze palsy Forced deviation | 0 1 2 |
| 3 | Best visual (visual fields) | No visual loss Partial hemianopia Complete hemianopia Bilateral hemianopia | 0 1 2 3 |
| 4 | Facial palsy (show teeth, raise brows, squeeze eyes shut) | Normal Minor Partial Complete | 0 1 2 3 |
| 5 | Motor arm left* (raise 90°, hold 10 seconds) | No drift Drift Cannot resist gravity No effort against gravity No movement | 0 1 2 3 4 |
| 6 | Motor arm right* (raise 90°, hold 10 seconds) | No drift Drift Cannot resist gravity No effort against gravity No movement | 0 1 2 3 4 |
| 7 | Motor leg left* (raise 30°, hold 5 seconds) | No drift Drift Cannot resist gravity No effort against gravity No movement | 0 1 2 3 4 |
| 8 | Motor leg right* (raise 30°, hold 5 seconds) | No drift Drift Cannot resist gravity No effort against gravity No movement | 0 1 2 3 4 |
| 9 | Limb ataxia (finger-nose, heel-shin) | Absent Present in 1 limb Present in 2 limbs | 0 1 2 |
| 10 | Sensory (pinprick to face, arm, leg) | Normal Partial loss Severe loss | 0 1 2 |
| 11 | Extinction/neglect (double simultaneous testing) | No neglect Partial neglect Complete neglect | 0 1 2 |
| 12 | Dysarthria (speech clarity to "mama, baseball, huckleberry, tip-top, fifty-fifty") | Normal articulation Mild to moderate dysarthria Near to unintelligible or worse | 0 1 2 |
| 13 | Best language** (name items, describe pictures) | No aphasia Mild to moderate aphasia Severe aphasia Mute | 0 1 2 3 |
| Total | - | 0-42 | |
| * For limbs with amputation, joint fusion, etc, score 9 and explain. ** For intubation or other physical barriers to speech, score 9 and explain. Do not add 9 to the total score. NIH Stroke Scale (PDF) | |||
Middle cerebral artery stroke
Middle cerebral artery (MCA) occlusions commonly produce the following:
- Contralateral hemiparesis
- Contralateral hypesthesia
- Ipsilateral hemianopsia
- Gaze preference toward the side of the lesion
- Agnosia
- Receptive or expressive aphasia, if the lesion occurs in the dominant hemisphere
- Neglect, inattention, and extinction of double simultaneous stimulation, with some nondominant hemisphere lesions
The MCA supplies the upper extremity motor strip. Consequently, weakness of the arm and face is usually worse than that of the lower limb.
Anterior cerebral artery stroke
Anterior cerebral artery (ACA) occlusions primarily affect frontal lobe function. Findings in ACA stroke may include the following:
- Disinhibition and speech perseveration
- Primitive reflexes (eg, grasping, sucking reflexes)
- Altered mental status
- Impaired judgment
- Contralateral weakness (greater in legs than arms)
- Contralateral cortical sensory deficits
- Gait apraxia
- Urinary incontinence
Posterior cerebral artery stroke
Posterior cerebral artery (PCA) occlusions affect vision and thought. Manifestations include the following:
- Contralateral homonymous hemianopsia
- Cortical blindness
- Visual agnosia
- Altered mental status
- Impaired memory
Vertebrobasilar artery occlusions are particularly difficult to localize, because they may cause a wide variety of cranial nerve, cerebellar, and brainstem deficits. These include the following:
- Vertigo
- Nystagmus
- Diplopia
- Visual field deficits
- Dysphagia
- Dysarthria
- Facial hypesthesia
- Syncope
- Ataxia
A hallmark of posterior circulation stroke is the presence of crossed findings: ipsilateral cranial nerve deficits and contralateral motor deficits. This contrasts with anterior stroke, which produces only unilateral findings.
Lacunar stroke
Lacunar strokes result from occlusion of the small, perforating arteries of the deep subcortical areas of the brain. The infarcts are generally from 2-20 mm in diameter. The most common lacunar syndromes include pure motor, pure sensory, and ataxic hemiparetic strokes. By virtue of their small size and well-defined subcortical location, lacunar infarcts do not lead to impairments in cognition, memory, speech, or level of consciousness.
Brain Imaging With CT Scanning and MRI
CT scanning
Imaging with CT scanning has multiple logistic advantages for patients with acute stroke. Image acquisition is faster with CT scanning than with MRI, allowing for assessment with an examination that includes noncontrast CT scanning, CT angiography (CTA), and CT perfusion scanning in a short amount of time. Expedient acquisition is of the utmost importance in acute stroke imaging because of the narrow window of time available for definitive ischemic stroke treatment with pharmacologic agents and mechanical devices.
CT scanning can also be performed in patients who are unable to tolerate an MR examination or who have contraindications to MRI, including implantable pacemakers, some aneurysm clips, or other ferromagnetic materials in their bodies. Additionally, CT scanning is more easily accessible for patients who require special equipment for monitoring and life support.
MRI
Conventional (spin echo) MRI may take hours to produce discernible findings in acute ischemic stroke. Diffusion-weighted imaging (DWI) is highly sensitive to early cellular edema, which correlates well with the presence of cerebral ischemia. For this reason, many centers include DWI in their standard brain MRI protocol. DWI MRI can detect ischemia much earlier than standard CT scanning or spin echo MRI can and provides useful data in patients with stroke or transient ischemic attack (TIA). (See the image below.)
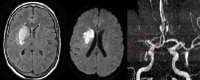 Magnetic resonance imaging (MRI) scan in a 70-year-old woman with a history of left-sided weakness for several hours. An axial T2 fluid-attenuated inversion recovery (FLAIR) image (left) demonstrates high signal in the lentiform nucleus with mass effect. The axial diffusion-weighted image (middle) demonstrates high signal in the same area, with corresponding low signal on the apparent diffusion coefficient (ADC) maps, consistent with true restricted diffusion and an acute infarction. Maximum intensity projection from a 3-dimensional (3-D) time-of-flight magnetic resonance angiogram (MRA, right) demonstrates occlusion of the distal middle cerebral artery (MCA) trunk (red circle).
Magnetic resonance imaging (MRI) scan in a 70-year-old woman with a history of left-sided weakness for several hours. An axial T2 fluid-attenuated inversion recovery (FLAIR) image (left) demonstrates high signal in the lentiform nucleus with mass effect. The axial diffusion-weighted image (middle) demonstrates high signal in the same area, with corresponding low signal on the apparent diffusion coefficient (ADC) maps, consistent with true restricted diffusion and an acute infarction. Maximum intensity projection from a 3-dimensional (3-D) time-of-flight magnetic resonance angiogram (MRA, right) demonstrates occlusion of the distal middle cerebral artery (MCA) trunk (red circle).
The most commonly used technique for perfusion MRI is dynamic susceptibility, which involves generating maps of brain perfusion by monitoring the first pass of a rapid bolus injection of contrast through the cerebral vasculature. Susceptibility-related T2 effects create signal loss in capillary blood vessels and parenchyma perfused by contrast.
Based on the central volume principle, dynamic brain perfusion data can be obtained. Cerebral blood volume (CBV), cerebral blood flow (CBF), and mean transit time (MTT) can be calculated using either perfusion MRI or CT scanning. (See the image below.)
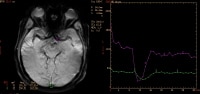 Regions of interest are selected for arterial and venous input (image on left) for dynamic susceptibility-weighted perfusion magnetic resonance imaging (MRI). Signal-time curves (image on right) obtained from these regions of interest demonstrate transient signal drop following the administration of intravenous contrast. The information obtained from the dynamic parenchymal signal changes postcontrast is used to generate maps of different perfusion parameters.
Regions of interest are selected for arterial and venous input (image on left) for dynamic susceptibility-weighted perfusion magnetic resonance imaging (MRI). Signal-time curves (image on right) obtained from these regions of interest demonstrate transient signal drop following the administration of intravenous contrast. The information obtained from the dynamic parenchymal signal changes postcontrast is used to generate maps of different perfusion parameters.
An evidence-based guideline from the American Academy of Neurology advises that DWI is more useful than noncontrast CT scanning for the diagnosis of acute ischemic stroke within 12 hours of symptom onset and should be performed for the most accurate diagnosis of acute ischemic stroke (level A). No recommendations were made regarding the use of perfusion-weighted imaging (PWI) in diagnosing acute ischemic stroke, as evidence to support or refute its value in this setting is insufficient.[70]
Intra-arterial contrast enhancement may be seen secondary to slow flow during the first or second day after onset of infarction. This finding has been correlated with increased infarct volume size.
Treatment and Management
The central goal of therapy in acute ischemic stroke is to preserve tissue in the ischemic penumbra, where perfusion is decreased but sufficient to stave off infarction. Tissue in this area of oligemia can be preserved by restoring blood flow to the compromised area and optimizing collateral flow.
Recanalization strategies, including the administration of intravenous (IV) recombinant tissue-type plasminogen activator (rt-PA) and intra-arterial approaches, attempt to establish revascularization so that cells in the penumbra can be rescued before irreversible injury occurs. Restoring blood flow can mitigate the effects of ischemia only if performed quickly.
Many surgical and endovascular techniques have been studied in the treatment of acute ischemic stroke. Carotid endarterectomy has been used with some success in the acute management of internal carotid artery occlusions, but no evidence supports its use acutely in ischemic stroke.
In addition to limiting the duration of ischemia, an alternative strategy is to limit the severity of ischemic injury (ie, neuronal protection). Neuroprotective strategies are intended to preserve the penumbral tissues and to extend the time window for revascularization techniques. At the present time, however, no neuroprotective agents have been shown to impact outcomes in ischemic stroke.
Palliative care
Palliative care is an important component of comprehensive stroke care. Some stroke patients will simply not recover, and others will be in a state of debilitation such that their comfort is the most humane and appropriate therapeutic concern. Some patients have advance directives providing instructions for medical providers in the event of severe medical illness or injury.
Clinical education
Prehospital care providers are essential to timely stroke care. Course curricula for prehospital care providers are beginning to include more information on stroke than ever before. Through certification and Acute Cardiac Life Support (ACLS) instruction, as well as continuing medical education classes, prehospital care providers can remain current on stroke warning signs, prehospital stroke tools, and triage protocols in their region, and can promote stroke awareness in their own communities.
Physician and nursing staff involved in the care of stroke patients, in the emergency department (ED) and in the hospital, should participate in scheduled stroke education. This will help them to maintain the skills required to treat stroke patients effectively and to remain current on medical advances for all stroke types.
Emergency Response and Transport
Recognition that a stroke may have occurred, activation of 911, and rapid transport to the appropriate receiving facility are necessary to provide stroke patients with the best chance for acute interventions. Of patients with signs or symptoms of stroke, 29-65% utilize some facet of the emergency medical services (EMS) system.
Most of the patients who call EMS are those who present within 3 hours of symptom onset. Calls to 911 and the use of EMS are associated with shorter time periods from symptom onset to hospital arrival.
Stroke should be a priority dispatch with prompt EMS response. EMS responders should provide advance notice to their ED destination in as timely a manner as possible so as to allow preparation and marshaling of personnel and resources. With the development of stroke center designation, which is currently in progress, such centers would then become the preferred destination for patients with acute stroke symptoms who utilize EMS.
Data supporting the use of emergency air transport for patients with acute stroke symptoms are limited. Further evaluation of this transportation modality is necessary to minimize the potentially high number of stroke mimics and to maximize the appropriate use of transport resources. Telemedicine is also a technology that has the potential to provide timely expert advice to rural and underserved clinics and hospitals.
Acute Management of Stroke
The goal for the emergent management of stroke is to assess the patient’s airway, breathing, and circulation (ABCs); stabilize the patient as necessary; and complete initial evaluation and assessment, including imaging and laboratory studies, within 60 minutes of patient arrival. A Finnish study demonstrated that time to treatment with fibrinolytics can be decreased with changes in EMS and ED coordination and in ED procedures for treating acute stroke patients.
A US study in which a multidisciplinary team used value stream analysis to assess the steps required to treat acute ischemic stroke with IV rt-PA found several inefficiencies in the protocol (eg, in patient routing) that were slowing treatment. Use of a revised protocol that targeted those inefficiencies reduced door-to-needle times from 60 to 39 minutes and increased the percentage of patients treated in 60 minutes or less after hospital arrival from 52% to 78%, with no change in symptomatic hemorrhage rate.
Critical decisions focus on the need for airway management, establishment of optimal blood pressure control, and identification of potential reperfusion therapies (IV fibrinolysis with rt-PA or intra-arterial approaches). Involvement of a physician with a special interest in stroke is ideal. Stroke care units with specially trained personnel exist and improve outcomes.
Comorbidities
Comorbid medical conditions also need to be addressed. Hyperthermia is infrequently associated with stroke but can increase morbidity. Administration of acetaminophen, by mouth or per rectum, is indicated in the presence of fever (temperature >100.4°F).
Oxygen supplementation
Supplemental oxygen is recommended when the patient has a documented oxygen requirement (ie, oxygen saturation < 95%). In the small proportion of patients with stroke who are relatively hypotensive, administration of IV fluid, vasopressor therapy, or both may improve flow through critical stenoses.
Hypoglycemia and hyperglycemia
Hypoglycemia needs to be identified and treated early in the evaluation. In contrast, the management of hyperglycemia in acute stroke remains an area of uncertainty.
Hyperglycemia is common after acute ischemic stroke, even in patients without diabetes. A Cochrane review found that the use of IV insulin to maintain serum glucose in the range of 4-7.5 mmol/L (72-135 mg/dL) in the first 24 hours of ischemic stroke did not improve functional outcome, death rates, or final neurologic deficit and significantly increased the risk of hypoglycemia.
Fibrinolytic Therapy
The only fibrinolytic agent that has been shown to benefit selected patients with acute ischemic stroke is rt-PA. While streptokinase may benefit patients with acute MI, in patients with acute ischemic stroke it has been shown to increase the risk of intracranial hemorrhage and death.
Fibrinolytics (ie, rt-PA) restore cerebral blood flow in some patients with acute ischemic stroke and may lead to improvement or resolution of neurologic deficits. Unfortunately, fibrinolytics may also cause symptomatic intracranial hemorrhage. Other complications include potentially hemodynamically significant hemorrhage and angioedema or allergic reactions.
Inclusion/exclusion criteria
Therefore, if the patient is a candidate for fibrinolytic therapy, a thorough review of the inclusion and exclusion criteria must be performed. The exclusion criteria largely focus on identifying risk of hemorrhagic complications associated with fibrinolytic use. The American Heart Association/American Stroke Association (AHA/ASA) inclusion guidelines for the administration of rt-PA are as follows :
- Diagnosis of ischemic stroke causing measurable neurologic deficit
- Neurologic signs not clearing spontaneously to baseline
- Neurologic signs not minor and isolated
- Symptoms not suggestive of subarachnoid hemorrhage
- No head trauma or prior stroke in past 3 months
- No myocardial infarction (MI) in past 3 months
- No gastrointestinal/genitourinary hemorrhage in previous 21 days
- No arterial puncture in a noncompressible site during the past 7 days
- No major surgery in past 14 days
- No history of prior intracranial bleeding
- Systolic blood pressure under 185 mm Hg, diastolic blood pressure under 110 mm Hg
- No evidence of acute trauma or bleeding
- Not taking an oral anticoagulant, or if so, international normalized ratio (INR) under 1.7
- If taking heparin within 48 hours, a normal activated prothrombin time (aPT)
- Platelet count of more than 100,000/μL
- Blood glucose greater than 50 mg/dL (2.7 mmol)
- No seizure with residual postictal impairments
- CT scan does not show evidence of multilobar infarction (hypodensity over one third hemisphere) or intracerebral hemorrhage
- The patient and family understand the potential risks and benefits of therapy
Whereas these inclusion/exclusion criteria are from the original FDA approval, subsequent data and experience have allowed some patients with what were previously considered relative contraindications to be safely treated. Involvement of a physician with stroke expertise is critical for assessing the risk/benefit consideration for these groups of patients.
Time to therapy
An rt-PA stroke study group from the National Institute of Neurologic Disorders and Stroke (NINDS) first reported that the early administration of rt-PA benefited carefully selected patients with acute ischemic stroke. The FDA subsequently approved the use of rt-PA in patients who met NINDS criteria. In particular, rt-PA had to be given within 3 hours of stroke onset and only after CT scanning had ruled out hemorrhagic stroke.
Subsequently, fibrinolytic therapy administered 3-4.5 hours after symptom onset was found to improve neurologic outcomes in the European Cooperative Acute Stroke Study III (ECASS III), suggesting a wider time window for fibrinolysis. On the basis of these and other data, in May 2009 the AHA/ASA revised the guidelines for the administration of rt-PA after acute stroke, expanding the window of treatment from 3 hours to 4.5 hours to provide more patients with an opportunity to benefit from this therapy.
Eligibility criteria for treatment during this later period are similar to those for earlier treatment but are more stringent, with any 1 of the following serving as an additional exclusion criterion:
- Age older than 80 years
- Use of oral anticoagulants, regardless of the INR
- Baseline score on the National Institutes of Health Stroke Scale (NIHSS) greater than 25
- History of stroke and diabetes
In a meta-analysis of nine major trials of thrombolysis treatment involving a total of 6756 patients with acute ischemic stroke, researchers found that administration of alteplase within 4.5 hours of stroke onset significantly improved outcomes, irrespective of age or stroke severity, with earlier treatment providing the greatest benefit. Good outcome was defined as modified Rankin score of 0 or 1, which indicates little or no residual disability at 3-6 months. The odds of a good stroke outcome were 75% higher for patients who received alteplase within 3 hours of symptom onset compared with those who did not. Patients given alteplase 3 to 4.5 hours after symptom onset had a 26% increased chance of a good outcome, and patients with a delay of more than 4.5 hours in receiving alteplase treatment had a nonsignificant 15% increase in the chance of a good recovery.
A 10-center European study of nearly 6900 patients found IV rt-PA to be most effective when given within 90 minutes of the onset of stroke symptoms. Patients scoring in the 7-12 range on the NIHSS had better outcomes when thrombolytic therapy was provided within 90 minutes of symptom onset than when it was provided 90-270 minutes after onset. For patients with minor stroke or moderate-to-severe stroke, however, treatment within the initial 90-minute window provided no additional advantage.
Hemorrhage risk
Although antiplatelet therapy may increase the risk for symptomatic intracerebral hemorrhage with fibrinolysis, a study by Diedler et al that included 3782 patients who had received 1 or 2 antiplatelet drugs found that the risk of intracerebral hemorrhage was small compared with the documented benefit of fibrinolysis. These researchers concluded that antiplatelet treatment should not be considered a contraindication to fibrinolysis, although caution is warranted in patients receiving the combination of aspirin and clopidogrel.
Data regarding the safety of fibrinolytic therapy in patients taking dabigatran, rivaroxaban, or apixaban are not available. Extreme caution should be used when considering fibrinolytic therapy in such patients.
Caution should also be exercised in the administration of rt-PA to patients with evidence of low attenuation (edema or ischemia) involving more than a third of the distribution of the middle cerebral artery (MCA) on their initial noncontrast CT scan; such patients are less likely to have a favorable outcome after fibrinolytic therapy and are at higher risk for hemorrhagic transformation of their ischemic stroke.
Ultrasound therapy
Researchers have studied the use of transcranial ultrasound as a means of assisting rt-PA in fibrinolysis. By delivering mechanical pressure waves to the thrombus, ultrasound can theoretically expose more of the thrombus’s surface to the circulating fibrinolytic agent. Further research is necessary to determine the exact role of transcranial Doppler ultrasound in assisting fibrinolytics in acute ischemic stroke.
Intra-arterial Reperfusion
There have been no completed human trials comparing intravenous versus intra-arterial administration of fibrinolytics. Theoretically, intra-arterial delivery may produce higher local concentrations of the fibrinolytic agent at lower total doses (and thus possibly lower the risk of a systemic bleed) and allow a longer therapeutic window. However, the longer time for initiating intra-arterial administration may mitigate some of this advantage.
The Interventional Management of Acute Stroke Study (IMS-III) was halted for futility after showing no additional benefit from intra-arterial therapies (rt-PA, mechanical thrombectomy, or both) compared with intravenous rt-PA in patients with large-vessel occlusions. Additional analyses of the IMS III data are under way to better understand the results and potentially identity subsets of patients who may benefit from the combined approach.
Intra-arterial fibrinolysis has been the traditional approach for patients with stroke from basilar artery occlusion. However, results of the Basilar Artery International Cooperation Study (BASICS), a prospective registry study in 592 patients, did not support unequivocal superiority of intra-arterial fibrinolysis over intravenous fibrinolysis.
A meta-analysis of case studies involving a total of 420 patients with basilar artery occlusion did indicate that recanalization was achieved more frequently with intra-arterial fibrinolysis than with intravenous fibrinolysis (65% vs 53%), but the report also found that death and long-term disability were equally common with the 2 techniques. These researchers concluded that intravenous fibrinolysis represents probably the best treatment that can be offered to these patients in hospitals without a 24-hour interventional neuroradiologic service.
Antiplatelet Agents
AHA/ASA guidelines recommend giving aspirin, 325 mg orally, within 24-48 hours of ischemic stroke onset. The benefit of aspirin is modest but statistically significant and appears principally to involve the reduction of recurrent stroke.
The International Stroke Trial and the Chinese Acute Stroke Trial (CAST) demonstrated modest benefit from the use of aspirin in the setting of acute ischemic stroke. The International Stroke Trial randomized 19,435 patients within 48 hours of stroke onset to treatment with aspirin 325 mg, subcutaneous heparin in 2 different dose regimens, aspirin with heparin, and a placebo. The study found that aspirin therapy reduced the risk of stroke recurrence within 14 days (2.8% vs 3.9%), with no significant excess of hemorrhagic strokes.
In CAST, which included 21,106 patients, aspirin treatment (160 mg/day) that was started within 48 hours of the onset of suspected acute ischemic stroke and was continued in hospital for up to 4 weeks reduced mortality to 3.3%, compared with 3.9% with placebo. A separate study also found that the combination of aspirin and low–molecular-weight heparin did not significantly improve outcomes.
Other antiplatelet agents have also been under evaluation for use in the acute presentation of ischemic stroke. In a preliminary pilot study, abciximab given within 6 hours showed a trend toward improved outcome at 3 months. However, the phase 3 Abciximab in Emergency Treatment of Stroke Trial (AbESTT-II) was terminated prematurely after 808 patients because of lack of efficacy and an increased rate of symptomatic or fatal intracranial hemorrhage in patients receiving abciximab.
Blood Pressure Control
Although hypertension is common in acute ischemic stroke and is associated with poor outcome, studies of antihypertensive treatment in this setting have produced conflicting results. A theoretical drawback of blood pressure reduction is that elevated blood pressure may counteract dysfunctional cerebral autoregulation from stroke, but limited evidence suggests that antihypertensive treatment in acute stroke does not change cerebral perfusion.[101]
Calcium channel blockers did not alter outcome after ischemic stroke in some trials. Possible adverse effects of antihypertensive treatment have been reported in certain trials, especially those using intravenous calcium channel blockers or oral beta blockers. In the Controlling Hypertension and Hypotension Immediately Post-Stroke (CHHIPS) trial, early lowering of blood pressure with labetalol and lisinopril slightly improved outcome and did not increase serious adverse events. However, CHHIPS had a small sample size.[102]
A study in 339 patients with ischemic stroke found that oral candesartan reduced combined vascular events but had no effect on disability.[101] However, the Scandinavian Candesartan Acute Stroke Trial (SCAST), a randomized, placebo-controlled, double-blind study involving 2029 patients, found no indication of benefit from candesartan but did find some suggestion of harm.[103]
In the single-blind, randomized China Antihypertensive Trial in Acute Ischemic Stroke (CATIS) study, which included 4,071 patients with acute ischemic stroke and elevated blood pressure, immediate blood pressure reduction with antihypertensive medication within 48 hours of symptom onset did not reduce the risk for death or major disability. CATIS excluded patients who received thrombolytic therapy. Mean systolic blood pressure was reduced from 166.7 to 144.7 mm Hg within 24 hours in the antihypertensive treatment group.[104, 105]
Among the 2,038 patients who received antihypertensive treatment, 683 reached the primary endpoint of death or major disability at 14 days or hospital discharge, compared with 681 of the 2,033 patients who received no antihypertensive treatment. At 3-month follow-up, 500 patients in the antihypertensive treatment group and 502 patients in the control group reached the secondary endpoint of death or major disability.[104, 105]
For patients who are not candidates for fibrinolytic therapy, current guidelines recommend permitting moderate hypertension in most patients with acute ischemic stroke. Most patients will experience spontaneous reduction in blood pressure over the first 24 hours without treatment.[86] The exceptions would be patients who have comorbidities (eg, aortic dissection, acute myocardial infarction [MI], decompensated heart failure, hypertensive emergency) that require emergent blood pressure management.
Thresholds for antihypertensive treatment in acute ischemic stroke patients who are not fibrinolysis candidates, according to the 2013 ASA guidelines, are systolic blood pressure higher than 220 mm Hg or diastolic blood pressure above 120 mm Hg.In those patients, a reasonable goal is to lower blood pressure by 15% during the first 24 hours after onset of stroke. Care must be taken to not lower blood pressure too quickly or aggressively, since this could worsen perfusion in the penumbra.
Mechanical Thrombectomy
Mechanical clot disruption is an alternative for patients in whom fibrinolysis is ineffective or contraindicated. Currently, 4 devices are approved by the FDA for the endovascular treatment of acute ischemic stroke, as follows:
- Merci Retriever (Concentric Medical, Mountain View, CA): Corkscrew-shaped device that captures and engages clots
- Penumbra System (Penumbra, Alameda, CA): Employs both aspiration and extraction
- Solitaire FR Revascularization Device (Covidien, Dublin, Ireland): Stent-retriever system; combines the ability to restore blood flow and retrieve clot
- Trevo (Concentric Medical, Mountain View, CA): Stent-retriever system
Successful recanalization occurred in 12 of 28 patients in the Mechanical Embolus Retrieval in Cerebral Ischemia (MERCI) 1 pilot trial, a study of the Merci Retrieval System. In a second MERCI study, recanalization was achieved in 48% of patients in whom the device was deployed. Clot was successfully retrieved from all major cerebral arteries; however, the recanalization rate for the MCA was lowest.
The Multi MERCI trial used the newer-generation Concentric retrieval device (L5). Recanalization was demonstrated in approximately 55% of patients who did not receive t-PA and in 68% of those to whom t-PA was given. Seventy-three percent of patients who failed intravenous t-PA therapy had recanalization following mechanical embolectomy. On the basis of these results, the FDA cleared the use of the MERCI device in patients who are either ineligible for or who have failed intravenous fibrinolytics.
In a trial of the Penumbra System in 23 patients who presented within 8 hours of symptom onset, revascularization to a Thrombolysis in Myocardial Infarction (TIMI) grade of 2 or 3 was accomplished in all 21 treated vessels. Vessel tortuosity prevented access by the device in 3 patients.
More recent trials of the stent-retriever systems demonstrated superiority in reperfusion over the original Merci systems. In the Solitaire Flow Restoration Device Versus the Merci Retriever in Patients with Acute Ischaemic Stroke (SWIFT) study, which enrolled 113 subjects with moderate or severe strokes within 8 hours after symptom onset, the Solitaire FR system demonstrated successful revascularization (TIMI 2-3 flow) in 61% of patients, compared with 24% of patients treated with the Merci system. Patients in the Solitaire FR group also had a higher rate of good 90-day clinical outcomes than did those in the Merci group (58% versus 33%, respectively).
A similar study, the Trevo Versus Merci Retrievers for Thrombectomy Revascularisation of Large Vessel Occlusions in Acute Ischaemic Stroke (TREVO 2) trial, reported successful reperfusion (TIMI 2-3 flow) in 86% of patients using the Trevor stent retriever, compared with 60% in the Merci group. The rate of good clinical outcomes at 90 days was also higher in the Trevo group than in the Merci group (40% vs 22%, respectively). Ongoing studies will better define the role of intra-arterial therapies with and without intravenous fibrinolysis.
Long-term results of the Stenting and Aggressive Medical Management for Preventing Recurrent Stroke in Intracranial Stenosis (SAMMPRIS) study confirm the superiority of aggressive medical management alone to aggressive medical management with stenting in patients with a stroke or transient ischemic attack resulting from stenosis of a major intracranial artery. Long-term follow-up results were available for 227 patients in the medical management group and 224 patients in the stenting group.
Occurrence rates for primary endpoint events (stroke or death within 30 days after enrollment or after either a revascularization procedure for the qualifying lesion during follow-up or a stroke in the territory of the qualifying artery beyond 30 days) in the medical group and the stenting group were 14.1% and 20.6%, respectively, at year 2 and 14.9% and 23.9% at year 3. Rates of any stroke and of any major hemorrhage were also significantly lower in the medical group than in the stenting group.
Fever Control
Antipyretics are indicated for febrile stroke patients, since hyperthermia accelerates ischemic neuronal injury. Substantial experimental evidence suggests that mild brain hypothermia is neuroprotective. The use of induced hypothermia is currently being evaluated in phase II clinical trials.
High body temperature in the first 12-24 hours after stroke onset has been associated with poor functional outcome. However, results from the Paracetamol (Acetaminophen) in Stroke (PAIS) trial did not support the routine use of high-dose acetaminophen (6 g daily) in patients with acute stroke, although post-hoc analysis suggested a possible beneficial effect on functional outcome in patients admitted with a body temperature of 37-39° C.
Cerebral Edema Control
Significant cerebral edema after ischemic stroke is thought to be somewhat rare (10-20%). Maximum severity of edema is reached 72-96 hours after the onset of stroke.
Early indicators of ischemia on presentation and on noncontrast CT (NCCT) scans are independent indicators of potential swelling and deterioration (see the image below). Mannitol and other therapies to reduce intracranial pressure (ICP) may be used in emergency situations, although their usefulness in swelling secondary to ischemic stroke is unknown. No evidence exists supporting the use of corticosteroids to decrease cerebral edema in acute ischemic stroke. Prompt neurosurgical assistance should be sought when indicated.
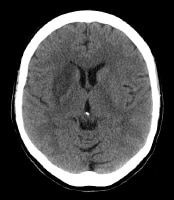 Axial noncontrast computed tomography (NCCT) scan demonstrates diffuse hypodensity in the right lentiform nucleus with mass effect upon the frontal horn of the right lateral ventricle in a 70-year-old woman with a history of left-sided weakness for several hours.
Axial noncontrast computed tomography (NCCT) scan demonstrates diffuse hypodensity in the right lentiform nucleus with mass effect upon the frontal horn of the right lateral ventricle in a 70-year-old woman with a history of left-sided weakness for several hours.
Patient position, hyperventilation, hyperosmolar therapy, and, rarely, barbiturate coma may be used, as in patients with increased ICP secondary to closed head injury. Hemicraniectomy has been shown to decrease mortality and disability among patients with large hemispheric infarctions associated with life-threatening edema.
The American Heart Association and the American Stroke Association have released a guideline for the management of cerebral and cerebellar infarction with brain swelling; recommendations include the following :
- Selected patients, including those able to handle an aggressive rehabilitation program, may benefit from decompressive craniectomy; younger patients may benefit most, and surgery is not recommended for patients older than 60 years
- Clinical evidence of deterioration in swollen supratentorial hemispheric ischemic stroke includes new or further impairment of consciousness, cerebral ptosis, and changes in pupillary size
- In patients with swollen cerebellar infarction, level of consciousness decreases because of brainstem compression; this decrease may include early loss of corneal reflexes and the development of miosis
- Standardized definitions are needed to facilitate studies of incidence, prevalence, risk factors, and outcomes
- Identification of high-risk patients should include both clinical and neuroimaging data
- Complex medical care of these patients includes airway management and mechanical ventilation, blood pressure control, fluid management, and glucose and temperature control
- In patients with swollen supratentorial hemispheric ischemic stroke, routine intracranial pressure monitoring or cerebrospinal fluid diversion is not indicated, but in patients who continue to deteriorate neurologically, decompressive craniectomy with dural expansion should be considered
- In patients with swollen cerebellar stroke who deteriorate neurologically, suboccipital craniectomy with dural expansion should be performed
- After a cerebellar infarct, performance of ventriculostomy to relieve obstructive hydrocephalus should be accompanied by decompressive suboccipital craniectomy to avoid deterioration from upward cerebellar displacement
- As many as one third of patients with swollen hemispheric supratentorial infarcts will be severely disabled and fully dependent on care even after decompressive craniectomy, whereas most patients with cerebellar infarct will have acceptable functional outcomes after surgery
Seizure Control
Seizures occur in 2-23% of patients within the first days after ischemic stroke. These seizures are usually focal, but they may be generalized. Although primary prophylaxis for poststroke seizures is not indicated, secondary prevention of subsequent seizures with standard antiepileptic therapy is recommended.
A fraction of patients who have experienced stroke develop chronic seizure disorders. Seizure disorders secondary to ischemic stroke should be managed in the same manner as other seizure disorders that arise as a result of neurologic injury.
Acute Decompensation
In the case of the rapidly decompensating patient or the patient with deteriorating neurologic status, reassessment of the ABCs as well as hemodynamics and reimaging are indicated. Many patients who develop hemorrhagic transformation or progressive cerebral edema will demonstrate acute clinical decline. Rarely, a patient may have escalation of symptoms secondary to increased size of the ischemic penumbra. Careful observation for hemorrhagic transformation (especially in the first 24 hours postreperfusion) and cerebral edema in patients with hemispheric or posterior fossa strokes in the first 24-36 hours is warranted.
Anticoagulation and Prophylaxis
Currently, data are inadequate to justify the routine use of heparin or other anticoagulants in the acute management of ischemic stroke. Patients with embolic stroke who have another indication for anticoagulation (eg, atrial fibrillation) may be placed on anticoagulation therapy nonemergently, with the goal of preventing further embolic disease; however, the potential benefits of that intervention must be weighed against the risk of hemorrhagic transformation.
Immobilized stroke patients in particular are at increased risk of developing deep venous thrombosis (DVT) and should receive early efforts to reduce the occurrence of DVT. The use of low-dose, subcutaneous unfractionated or low–molecular-weight heparin may be appropriate in these cases. The CLOTS (Clots in Legs Or sTockings after Stroke) trial demonstrated that intermittent pneumatic compression of the lower extremities, started in the first 3 hospital days, reduced the risk of DVT in immobile patients with acute stroke.
Neuroprotective Agents
The rationale for the use of neuroprotective agents is that reducing the release of excitatory neurotransmitters by neurons in the ischemic penumbra may enhance the survival or these neurons. Despite very promising results in several animal studies, however, no single neuroprotective agent in ischemic stroke has as yet been supported by randomized, placebo-controlled human studies. Nevertheless, substantial research is under way evaluating different neuroprotective strategies.
Hypothermia is fast becoming the standard of care for the ongoing treatment of patients surviving cardiac arrest from ventricular tachycardia or ventricular fibrillation. However, no major clinical study has demonstrated a role for hypothermia in the early treatment of ischemic stroke.
Stroke Prevention
Primary prevention refers to the treatment of individuals with no history of stroke. Measures may include the use of platelet antiaggregants, statins, and exercise. The 2011 AHA/ASA guidelines for the primary prevention of stroke emphasize the importance of lifestyle changes to reduce well-documented modifiable risk factors, citing an 80% lower risk of a first stroke in people who follow a healthy lifestyle compared with those who do not.
Secondary prevention refers to the treatment of individuals who have already had a stroke. Measures may include the use of platelet antiaggregants, antihypertensives, statins, and lifestyle interventions. A study by the Warfarin-Aspirin Symptomatic Intracranial Disease Trial Investigators concluded that in stroke patients who have significant intracranial arterial stenosis, aspirin should be used in preference to warfarin for secondary prevention.
Smoking cessation, blood pressure control, diabetes control, a low-fat diet, weight loss, and regular exercise should be encouraged as strongly as the medications described above. The 2011 AHA/ASA guidelines recommend ED-based smoking cessation interventions, and consider it reasonable for EDs to screen patients for hypertension and drug abuse.
Written prescriptions for exercise and medications for smoking cessation (ie, nicotine patch, bupropion, varenicline) increase the likelihood of success with these interventions. In addition, the 2011 AHA/ASA guidelines for primary stroke prevention indicate that it is reasonable to avoid exposure to environmental tobacco smoke, despite a lack of stroke-specific data.
Aspirin for primary prevention
Overall, the value of aspirin in primary prevention appears uncertain, and its use for this purpose is not recommended for patients at low risk. Aspirin is recommended for primary prevention only in persons with at least a 6-10% risk of cardiovascular events over 10 years.
On the other hand, low-dose aspirin may be beneficial for primary prevention of stroke in women. A randomized, placebo-controlled trial in 39,876 initially healthy women aged 45 years or older demonstrated that 100 mg of aspirin on alternate days resulted in a 24% reduction in the risk of ischemic stroke, with a nonsignificant increase in the risk of hemorrhagic stroke.
Secondary prevention guidelines
Guidelines issued in 2014 by the American Heart Association (AHA)/American Stroke Association (ASA) on the secondary prevention of stroke emphasize nutrition and lifestyle and include a new section on aortic atherosclerosis. New recommendations include the following :
- Patients who have had a stroke or transient ischemic attack (TIA) should be screened for diabetes and obesity
- Patients should possibly be screened for sleep apnea
- Patients should possibly undergo a nutritional assessment and be advised to follow a Mediterranean-type diet
- Patients who have had a stroke of unknown cause should undergo long-term monitoring for atrial fibrillation (AF)
- The new oral anticoagulants dabigatran (class I, level of evidence [LOE] A), apixaban (class I, LOE B), and rivaroxaban (class IIa, LOE B) are among the drugs recommended for patients with nonvalvular AF
Based on research results, the guidelines also recommend that, in patients without deep venous thrombosis (DVT), a patent foramen ovale not be closed. In addition, because there is little data to suggest that niacin or fibrate drugs, as a means to raise high-density lipoprotein (HDL) cholesterol, reduce secondary stroke risk, the guidelines no longer recommend their use.
Dual antiplatelet therapy for secondary prevention
A systematic review and meta-analysis of 12 randomized trials involving 3766 patients concluded that, compared with aspirin alone, dual antiplatelet therapy with aspirin plus either dipyridamole or clopidogrel appears to be safe and effective in reducing stroke recurrence and other vascular events (ie, transient ischemic attack [TIA], acute coronary syndrome, MI), in patients with acute ischemic stroke or TIA. Dual therapy was also associated with a nonsignificant trend toward increased major bleeding.
The European/Australasian Stroke Prevention in Reversible Ischemia Trial (ESPRIT) showed that the combination of aspirin and dipyridamole was preferable to aspirin alone as antithrombotic therapy for cerebral ischemia of arterial origin In ESPRIT, secondary prevention was started within 6 months of a TIA or minor stroke of presumed arterial origin.
The addition of extended-release dipyridamole to aspirin therapy appears to be equally safe and effective whether started early or late after stroke. A German study in 543 patients found no significant difference in disability at 90 days, regardless of whether dipyridamole was started within 24 hours of stroke or TIA onset or after 7 days of aspirin monotherapy.
In contrast, the Management of AtheroThrombosis with Clopidogrel in High-risk patients with recent transient ischaemic attack or ischaemic stroke (MATCH) trial, which included 7599 patients, found that adding aspirin to clopidogrel did not significantly reduce major vascular events. However, the risk of life-threatening or major bleeding was increased by the addition of aspirin.
Carotid artery stenosis
For patients at risk for stroke from asymptomatic carotid artery stenosis, the 2011 AHA/ASA primary prevention guidelines state that older studies that showed revascularization surgery as more beneficial than medical treatment may now be obsolete because of improvements in medical therapies. Therefore, individual patient comorbidities, life expectancy, and preferences should determine whether medical treatment alone or carotid revascularization is selected.
Atrial fibrillation
Atrial fibrillation (AF) is a major risk factor for stroke. The 2011 AHA/ASA primary stroke prevention guideline recommends that EDs screen for AF and assess patients for anticoagulation therapy if AF is found.
In the Atrial fibrillation Clopidogrel Trial with Irbesartan for prevention of Vascular Events (ACTIVE W), oral anticoagulation with warfarin proved superior to clopidogrel plus aspirin for prevention of vascular events in patients with AF who were at high risk of stroke. The study was stopped early because of clear evidence of superiority of oral anticoagulation therapy.
Interestingly, in ACTIVE W, the rate of vascular events was significantly higher in patients who switched from warfarin to clopidogrel plus aspirin as a result of randomization than in patients who had been on warfarin before study enrollment and remained on warfarin during the study. The benefit of anticoagulation therapy over dual antiplatelet therapy was much more modest in patients who had not been on warfarin before study initiation and were then randomized to warfarin.
The 2011 ACC Foundation (ACCF)/AHA/Heart Rhythm Society (HRS) AF guideline update states that the new anticoagulant dabigatran is useful as an alternative to warfarin in patients with AF who do not have a prosthetic heart valve or hemodynamically significant valve disease.However, a 2012 meta-analysis found an increased risk for MI or acute coronary syndrome with dabigatran.
For patients with AF after stroke or TIA, the 2010 AHA/ASA secondary stroke prevention guidelines are in accord with the standard recommendation of warfarin, with aspirin as an alternative for patients who cannot take oral anticoagulants. However, clopidogrel should not be used in combination with aspirin for such patients, because the bleeding risk of the combination is comparable to that of warfarin. The guideline states that the benefit of warfarin after stroke or TIA in patients without AF has not been established.
Specialized Stroke Centers
The concept of the specialized stroke center has evolved in response to the multitude of factors involved in the care of patients with acute stroke. The Brain Attack Coalition provided recommendations for the establishment of 2 tiers of stroke centers: primary stroke centers (PSCs) and comprehensive stroke centers (CSCs). The Joint Commission for the Accreditation of Hospital Organizations (JCAHO) now provides accreditation for PSCs and CSCs. These centers are characterized as follows:
- PSC: Designed to maximize the timely provision of stroke-specific therapy, including the administration of rt-PA; the center is also capable of providing care to patients with uncomplicated stroke
- CSC: Shares the commitment that the PSC has to acute delivery of rt-PA and also provides care to patients with hemorrhagic stroke and intracranial hemorrhage, as well as to all patients with stroke who require emergent advanced imaging, intra-arterial therapies, neurosurgical interventions, and management in a neurosurgical intensive care unit (NSICU)
PSCs and CSCs work most effectively when integrated into a regional stroke system of care so that patients are treated at the most appropriate hospital based on factors such as severity, comorbidities, and timing. Integrating regional prehospital services (911 and EMS) into this system of care ensures the most appropriate triage from the field.
Additionally, stroke centers should have personnel versed in the monitoring of stroke vital signs, which include the following:
- Blood pressure
- Glucose levels
- Temperature
- Oxygenation
- Change in neurologic status
A further tier, acute stroke ready hospitals, is being defined as hospitals in which most of the necessary resources are in place to emergently evaluate patients and potentially treat them with fibrinolytics, with the assistance of remote stroke expertise, typically by telemedicine. Key to the optimal function of these stroke centers is their interactions within a regional stroke system of care.
Coordination of care
Once patients have been identified as potential stroke patients, their ED evaluation must be fast-tracked to allow for the completion of required laboratory tests and requisite noncontrast head CT scanning, as well as for the notification and involvement of neurologic consultants. These requirements have led to the development of "code stroke" protocols for the ED. In addition, EMS personnel are trained to identify possible stroke patients and arrange for their speedy, preferential transport to a PSC or CSC.
Hospitals with specialized stroke teams have demonstrated significantly increased rates of fibrinolytic administration and decreased mortality. Cumulatively, the center should identify performance measures and include mechanisms for evaluating the effectiveness of the system, as well as its component parts. The acute care of the stroke patient is more than anything a systems-based team approach requiring the cooperation of the ED, radiology, pharmacy, neurology, and intensive care unit (ICU) staff.
A stroke system should ensure effective interaction and collaboration among the agencies, services, and people involved in providing prevention and the timely identification, triage to the most appropriate hospital, rapid transport, treatment, and rehabilitation of stroke patients. For more information,
Consultations
A stroke team or an experienced professional who is sufficiently familiar with stroke should be available within 15 minutes of the patient's arrival in the ED. Other consultations are tailored to individual patient needs. Often, occupational therapy, physical therapy, speech therapy, and physical medicine and rehabilitation experts are consulted within the first day of hospitalization.
Consultation of cardiology, vascular surgery, or neurosurgery may be warranted based on the results of carotid duplex scanning , neuroimaging, transthoracic and transesophageal echocardiography, and clinical course. During hospitalization, additional useful consultations include the following:
- Home health care coordinator
- Rehabilitation coordinator
- Social worker
- Psychiatrist (commonly for depression)
- Dietitian
Medication Summary
While only 1 drug, recombinant tissue-type plasminogen activator (rt-PA), has demonstrated efficacy and effectiveness in treating acute ischemic stroke and is approved by the FDA, other medications are equally important. National consensus panels have included the use of antihypertensives, anticonvulsants, and osmotic agents in their recommendations. Additional agents may be required for comorbid illnesses in many patients with stroke.
Medications for the management of ischemic stroke can be distributed into the following categories:
- Anticoagulation
- Reperfusion
- Antiplatelet
- Neuroprotective
 RSS Feed
RSS Feed Twitter
Twitter


 Sunday, March 29, 2015
Sunday, March 29, 2015
 Unknown
Unknown

 Posted in
Posted in
Herbs with Maxs Ace is the most appropriate alternative treatment obat stroke ringan paling ampuh
ReplyDeletePatients do the diet, as mentioned earlier, namely apples for 5 consecutive days. cara pengobatan batu empedu tanpa operasi
HOW i got a cure for stroke
ReplyDeleteI really want to take this time out to show my appreciation to a doctor who advised me correctly. I had stroke for 12 years and my full left side was paralyzed because of this i could say i was half human because i could not function where others are functioning. I knew i needed help and i began to search and i saw his contact from a testimony some people were giving, i never believed because i have tried so many medicines and therapies yet no cure. I still contacted him to see what he had to offer. I got the medicine (herbal medication) he told me about and i could not believe my eyes. In a matter of 3 months i was completely okay. If you have any stroke related illness or paralysis feel free to reach him for info and cure on (josephalberteo@gmail.com) he treated me and he can treat you too. cheers.
I started on COPD Herbal treatment from Ultimate Health Home, the treatment worked incredibly for my lungs condition. I used the herbal treatment for almost 4 months, it reversed my COPD. My severe shortness of breath, dry cough, chest tightness gradually disappeared. Reach Ultimate Health Home via their email at ultimatehealthhome@gmail.com . I can breath much better and It feels comfortable!
ReplyDelete